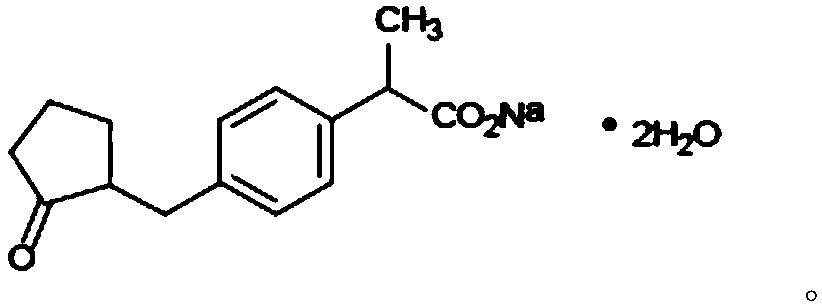
Loxoprofen sodium cataplasm
A technology of cataplasms and fillers, applied in the field of loxoprofen sodium cataplasms, which can solve the problems of lack of delayed hypersensitivity reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
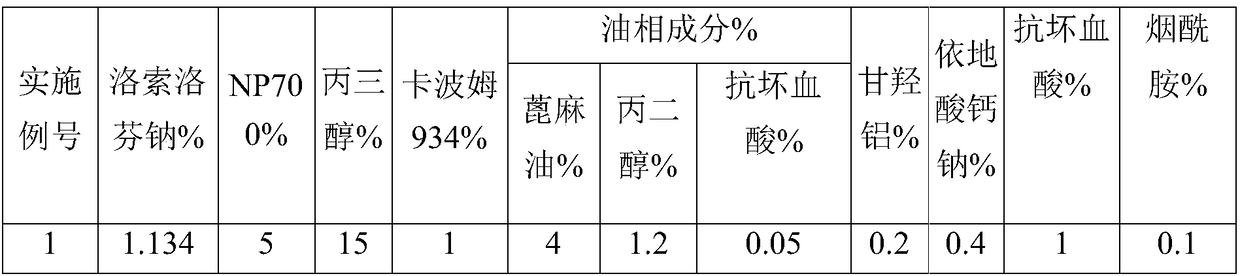
Embodiment 1
[0026] Pharmacological Example 1 In Vitro Release Experiment
[0027] According to the method provided in the third method (paddle and disk method, used for transdermal patch) in the second appendix XD of the Chinese Pharmacopoeia 2010 edition, the emergency release of the patch obtained in Examples 1 to 6 is measured . The specific method is as follows
[0028] In the test, physiological saline was used as the release medium, and the release medium was added into the dissolution cup, pre-warmed to (32±0.5°C) to remove the protective layer of the cataplasm, cut into a size of 2.5cmx7.5cm, and put it flat into a dialysis bag (molecular weight cut-off 14,000 ), with the release side facing up, placed between two layers of discs, so that the edges of the disc clamp the two ends of the dialysis bag, and then wrap and fix with rubber bands to fix the disc. At 10min, 20min, 30min, 45min, 60min, 90min, 2h, 2.5h, 3h and 4h, 6mL samples were taken from the dissolution vessel respecti...
Embodiment 2
[0029] Pharmacological Example 2 New Zealand Rabbit Animal Model Skin Irritation Experiment of Delayed Hypersensitivity
[0030] 1. Experimental animals: select New Zealand white rabbits, weighing 2.0-2.5 kg, male or female, 10 rabbits in each group.
[0031] 2. Modeling, the model group uses potassium dichromate (K 2 Cr 2 o 7 ) for immunization, each rabbit was injected intramuscularly with 1% K 2 Cr 2 o 7 + Freund's complete adjuvant mixture (volume ratio 1:1) 0.5ml. At the same time, 0.1ml of the mixed solution was injected intradermally in 4 places, and after continuous injection for 7 days, the back was treated with K 2 Cr 2 o 7 / 40% dimethyl sulfoxide solution for skin application. In the third week after the first injection, 0.5ml of 0.5% K2Cr2O7 solution was injected intramuscularly to strengthen the hypersensitivity reaction.
[0032] 3. Experimental method
[0033] After the last injection, cut off most of the rabbit hair on both sides of the spine with sc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com