Sinonovacula-constricta I-type lysozyme-2 genes, encoded protein and construction method of recombinant sinonovacula-constricta I-type lysozyme-2 gene engineering bacteria
A technology of genetically engineered bacteria and encoded proteins, applied in genetic engineering, plant genetic improvement, peptide/protein components, etc., can solve food safety problems, restrict the survival and growth of razor clams, and increase marine pollution, etc., and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0045] Cloning and sequence analysis of type I lysozyme-2 gene
[0046] (1) Through the previous EST (Expressed Sequence Tag) analysis of the cDNA library of Vibrio parahaemolyticus-induced razor clams, an EST sequence encoding the type I lysozyme-2 gene was found, and the sequencing analysis of this EST clone showed that the clone A partial fragment encoding razor clam type I lysozyme-2;
[0047] (2) RACE primer design: 3' RACE nested primers were designed according to the EST clone encoding a partial fragment of the razor clam type I lysozyme-2 gene: 3' upstream specific amplification primer 1: CAGTTCCTCCCGGACCTGTTGA, 3' upstream specific amplification Amplified primer 2: GCCAGACGTACGCTAGGGAACAC, amplified 3' adapter primer Adaptor3: GGCCACGCGTCGACTAGTACTT;
[0048] (3) Using 3’RACE amplification to obtain the full-length sequence of the razor clam type I lysozyme-2 gene, the specific steps are as follows:
[0049] a. Total RNA extraction: Take the hepatopancreas tissue (...
specific Embodiment 2
[0053] Construction and Expression of Type I Lysozyme-2 Genetic Engineering Bacteria
[0054] a. Total RNA extraction: Take the hepatopancreas tissue (0.2 g-1 g) of razor clam constrictor into a 1.5 mL RNA free centrifuge tube, add 1.0 mL of Trizol reagent (purchased from Takara Company), and fully homogenize with a homogenizer. Centrifuge at 12000 g at 4 °C for 5 min, take the supernatant, add 0.2 mL of chloroform, shake and mix, let stand at room temperature for 5 min, centrifuge at 12000 g at 4 °C for 15 min, draw the supernatant into a centrifuge tube, add the Add an equal volume of isopropanol to the supernatant, mix well, let stand at room temperature for 5 min, centrifuge at 12,000 rpm for 5 min at 4 °C, remove the supernatant, add 1 mL of ethanol with a mass percentage concentration of 75% to the precipitate, 4 °C, Centrifuge at 12,000 rpm for 5 min, discard the supernatant, add 1 mL of 75% ethanol to the pellet to resuspend the pellet, centrifuge at 12,000 rpm for 5 ...
specific Embodiment 3
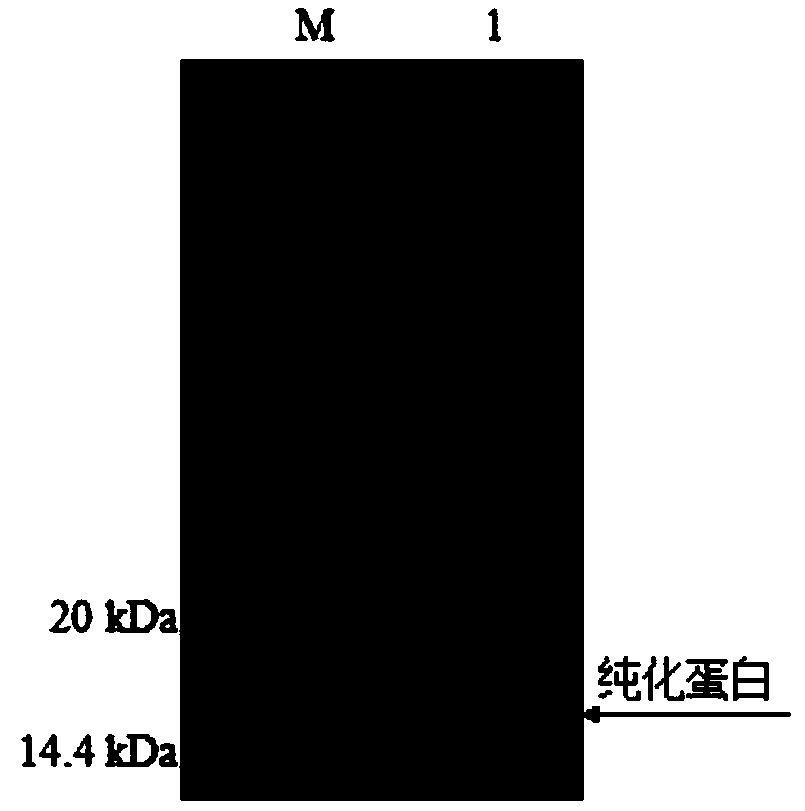
[0061] Purification of recombinant proteins
[0062] a. Bacteria lysis: resuspend 100 mL of bacterial sediment in 10 mL lysis buffer (imidazole concentration: 5 mM), add 1 mg / mL lysozyme, incubate on ice for 30 min, and ultrasonically disrupt the bacteria (ultrasonic 5 s, stop for 10 s, a total of 6 times, power 30w, 2 min), 10000 g, 4 ℃, centrifuge 20 min, collect the supernatant.
[0063] b. Protein purification: draw 1 mL Ni-NTA Sefinose TM Put Resin on the column, wash twice with sterile water, and then equilibrate once with lysis buffer (imidazole concentration 5mM); mix the supernatant with the Ni-NTA Sefinose that was previously loaded TM Resin was mixed, mixed for 2 h at 4 ℃, the effluent was collected, and washed buffer (imidazole concentration 40 mM), eluted twice each time with 10 mL, the effluent was collected separately, and 1.25 mL elution buffer (imidazole concentration 250 mM) was added , eluted 4 times, and the effluents were collected respectively. Take th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com