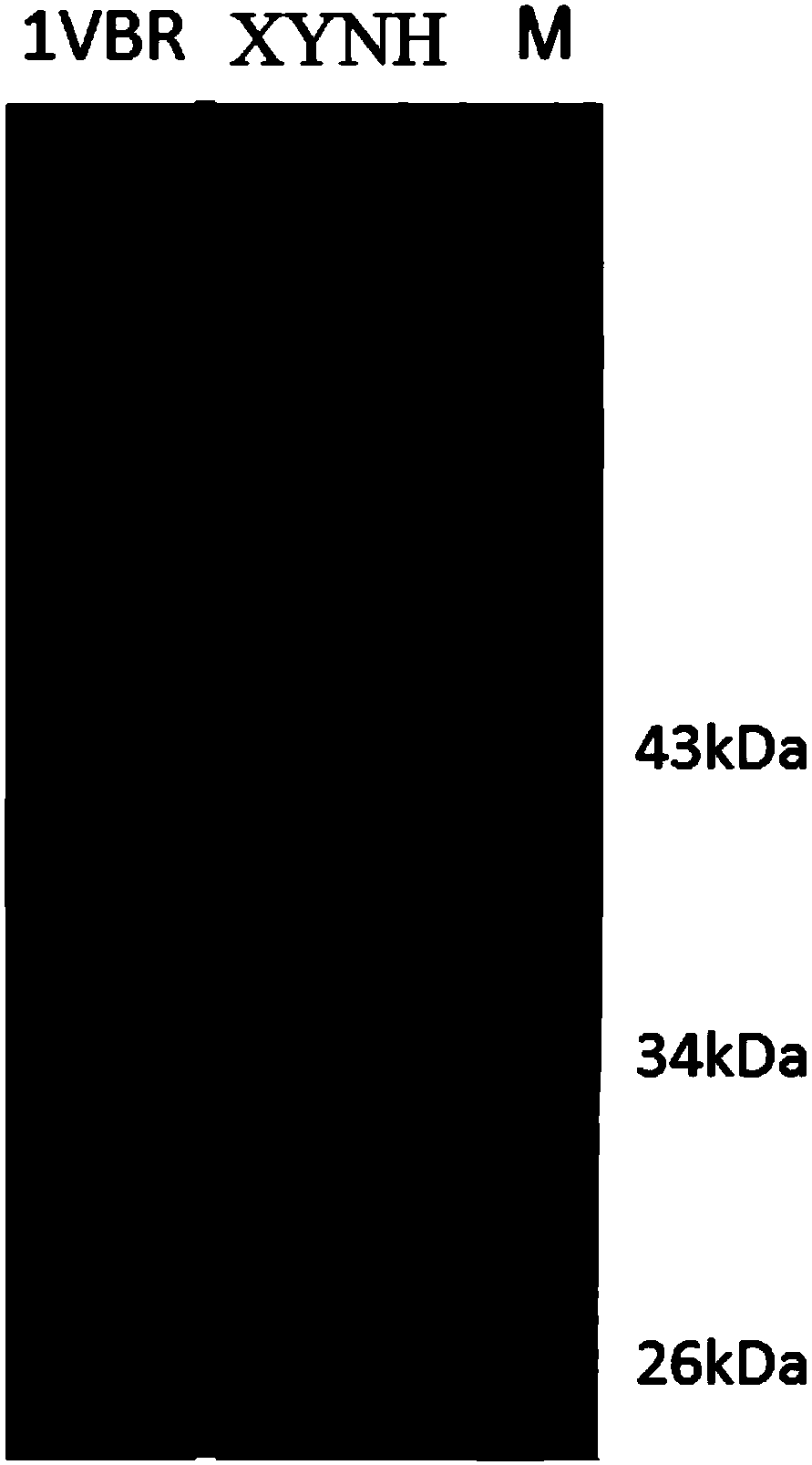
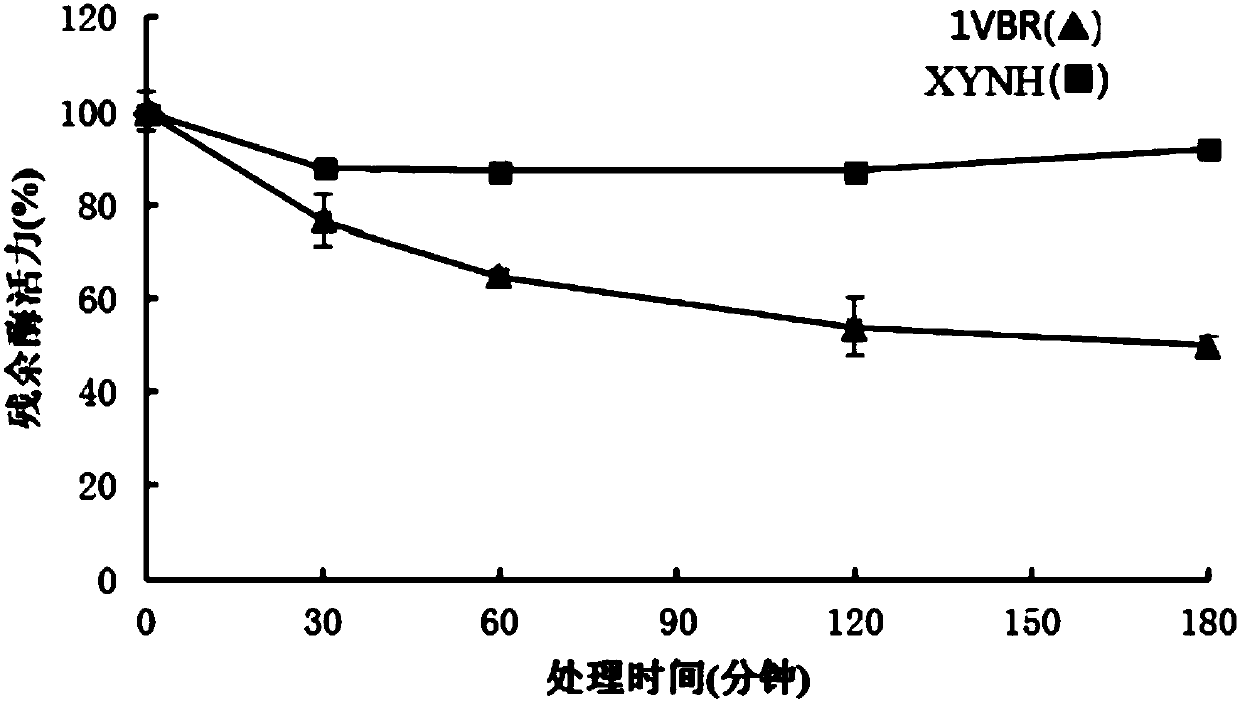
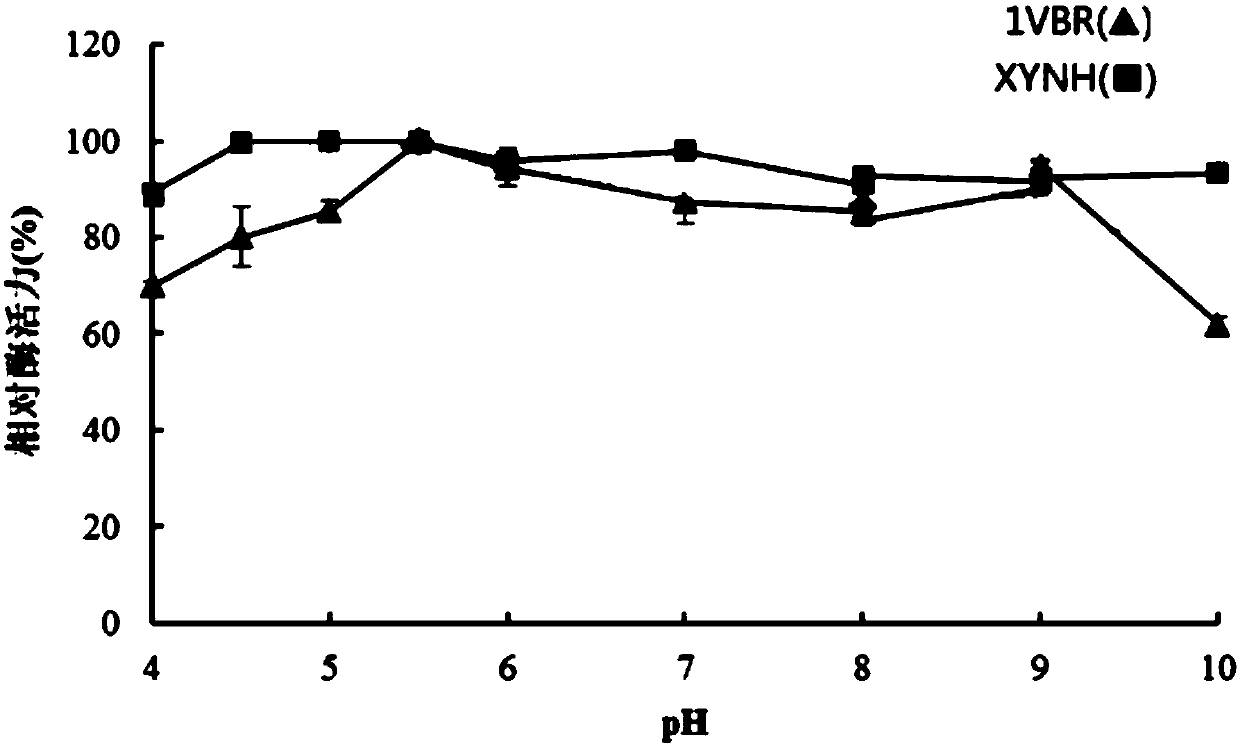
Mutant XYNH of extreme heat-resistant xylanase 1VBR and use thereof
A technology of xylanase and mutants, which is applied in the fields of protein engineering and genetic engineering, can solve problems such as impact, unfavorable product quality, and increased production costs, and achieve the effects of improving stability, good adaptability, and strong stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Obtaining of XYNH gene sequence of xylanase mutant
[0032] According to sequence comparison, the amino acid sequence of xylanase 1VBR from Thermotoga maritima MSB8 published in PDB and the predicted xylanase (EHA58720.1 ) have an amino acid sequence similarity of 100%. The predicted xylanase gene sequence is a gene sequence (873,589→874,572 bp) in the genome sequence of Thermotoga maritima MSB8.
[0033] Based on the codon usage preference of Pichia pastoris, the rare codons were converted to high-frequency expression codons, and the predicted xylanase (EHA58720.1) gene sequence was compared with the amino acid sequence of xylanase 1VBR Codon optimization was carried out to obtain the optimized xylanase 1VBR gene sequence. Based on this template, the 290th amino acid in the amino acid sequence of xylanase 1VBR was changed from phenylalanine to histidine by gene site-directed mutagenesis to obtain the gene sequence of xylanase mutant XYNH, As shown in S...
Embodiment 2
[0034] Example 2: Construction of recombinant expression plasmid pPIC9K-XYNH containing xylanase mutant XYNH gene
[0035] The terminator sequence preferred by Pichia pastoris was added to the 3' end of the xylanase mutant XYNH gene, and restriction endonuclease EcoR I and Not I sites were introduced at the 5' end and 3' end respectively, and the gene sequence was Hand over to Wuhan Qingke Innovation Biotechnology Co., Ltd. to complete the whole gene synthesis. The optimized xylanase mutant XYNH gene and the secreted expression vector pPIC9K were digested with restriction endonucleases EcoR I and Not I, and then the two were connected with ligase to construct the recombinant expression plasmid pPIC9K-XYNH. The recombinant expression plasmid was transformed into Escherichia coli DH5α competent cells, and the positive clone strain pPIC9K-XYNH-DH5α was screened out by PCR verification method.
Embodiment 3
[0036] Example 3: Construction of Pichia pastoris genetically engineered strains that efficiently secrete and express xylanase mutant XYNH
[0037] The LB liquid medium was used to activate the cultured strain pPIC9K-XYNH-DH5α, and the recombinant plasmid pPIC9K-XYNH was extracted. The recombinant plasmid was linearized with restriction endonuclease Bgl II, and the digested product was recovered. Refer to EasySelect TM PichiaExpression Kit prepared Pichia GS115 competent cells. Take about 10 μg of linearized plasmid and 80 μL of competent cells, mix gently, place on ice for 15 min, transfer to a pre-cooled 0.2 cm electroporation cuvette, add 1 mL of pre-cooled 1 mol / L sorbitol immediately after the 1500V electric shock, and Let it stand in a 30°C incubator for 1 hour, spread it on an MD plate, and incubate it upside down at 30°C for about 48 hours until transformants appear.
[0038] Pick a single colony and inoculate them on MD plates containing 0.25, 0.5, 1.0, 2.0, 3.0 a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com