Preparing methods of p-aminophenyl-beta-hydroxyethylsulfonyl and p-minophenyl-beta-hydroxyethylsulfonyl sulphonate
A technology of p-aminophenyl and hydroxyethyl sulfone, which is used in the synthesis of reactive dye intermediates, can solve the problems of palladium-carbon catalysts, such as easy sulfur poisoning price, low production efficiency, and low catalytic selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
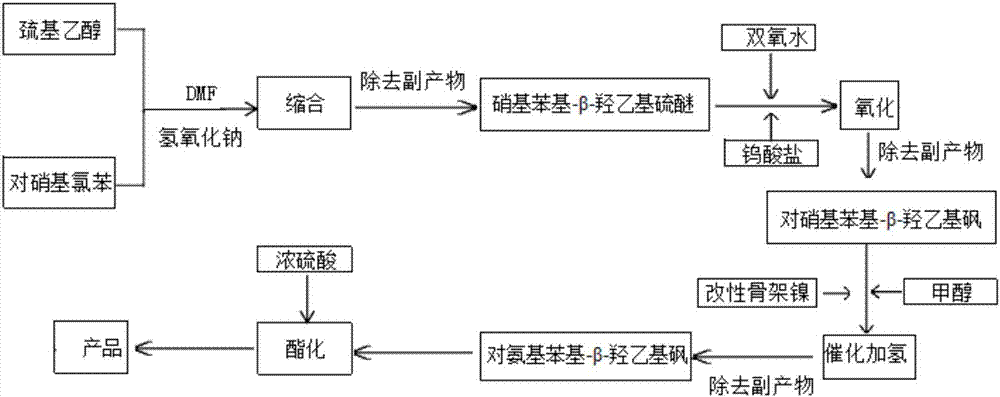
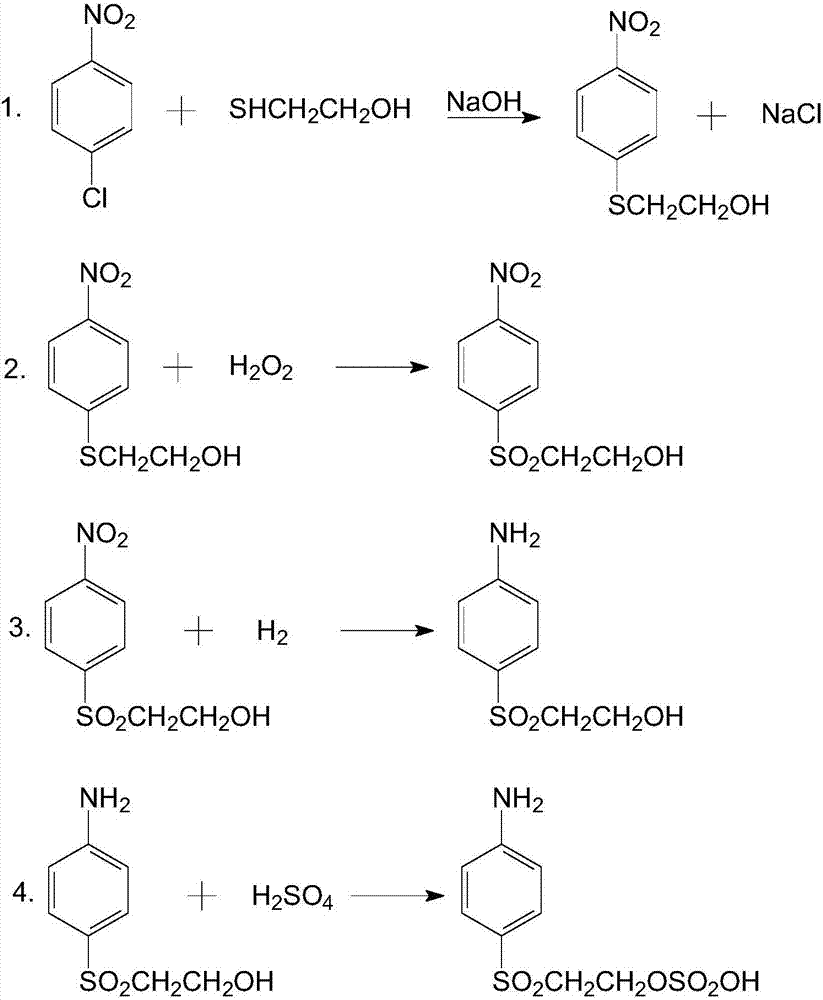
[0070] A preparation method of p-aminophenyl-β-hydroxyethyl sulfone, the steps are as follows:
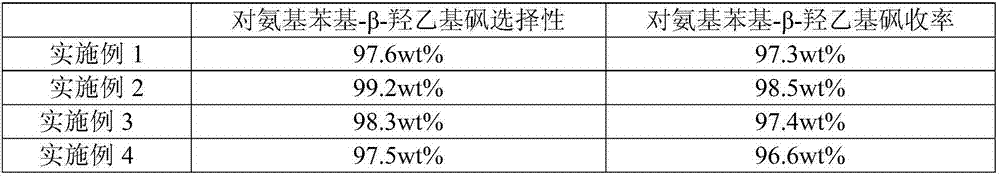
[0071] The solid oxidizing material p-nitrophenyl-β-hydroxyethyl sulfone, methyl alcohol and modified framework nickel are added in the hydrogenation autoclave, and the mass ratio of solid oxidizing material, methanol and modified framework nickel is 1:4: 0.05, the purity of methanol is 99.99wt%, and then carry out hydrogenation reaction at 70°C and hydrogen pressure of 1.5MPa for 4h; Hydrogen product p-aminophenyl-β-hydroxyethyl sulfone;
[0072] The modified skeleton nickel uses nickel as the skeleton, including: 85 parts by mass of Ni, 15 parts by mass of Al, and 4 parts by mass of metal additive; the metal additive is Mo; it is prepared by the following method:
[0073] (1) Melt down in an inert atmosphere after metal additive is mixed with the nickel-aluminum alloy powder that contains nickel 85wt%, obtain alloy, continue roasting 3h in melting furnace, with 0.5 * 10 6 After...
Embodiment 2
[0076] A preparation method of p-aminophenyl-β-hydroxyethyl sulfone, the steps are as follows:
[0077] The solid oxidizing material p-nitrophenyl-β-hydroxyethyl sulfone, methyl alcohol and modified framework nickel are added in the hydrogenation autoclave, and the mass ratio of solid oxidizing material, methanol and modified framework nickel is 1:4: 0.03, the purity of methanol is 99.99wt%, and then the hydrogenation reaction is carried out at 85°C and the hydrogen pressure is 2.0MPa for 3.5h; Hydrogenation product p-aminophenyl-β-hydroxyethyl sulfone;
[0078] Wherein the modified skeleton nickel uses nickel as the skeleton, including: 90 parts by mass of Ni, 10 parts by mass of Al, and 8 parts by mass of metal additives; the metal additive is Cu; prepared by the following method:
[0079] (1) Melt down in an inert atmosphere after metal additive is mixed with the nickel-aluminum alloy powder that contains nickel 90wt%, obtain alloy, continue roasting 4h in melting furnace,...
Embodiment 3
[0082] A preparation method of p-aminophenyl-β-hydroxyethyl sulfone, the steps are as follows:
[0083] The solid oxidizing material p-nitrophenyl-β-hydroxyethyl sulfone, methyl alcohol and modified framework nickel are added in the hydrogenation autoclave, and the mass ratio of solid oxidizing material, methanol and modified framework nickel is 1:4: 0.02, the purity of methanol is 99.99wt%, and then hydrogenation reaction is carried out at 100°C and hydrogen pressure of 2.5MPa for 3h; Hydrogen product p-aminophenyl-β-hydroxyethyl sulfone;
[0084] Wherein the modified skeleton nickel uses nickel as the skeleton, including: 80 parts by mass of Ni, 20 parts by mass of Al, and 5 parts by mass of metal additive; the metal additive is Cr; prepared by the following method:
[0085] (1) Melt down in an inert atmosphere after metal additive is mixed with the nickel-aluminum alloy powder that contains nickel 80wt%, obtain alloy, continue roasting 3h in melting furnace, with 0.8 * 10 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com