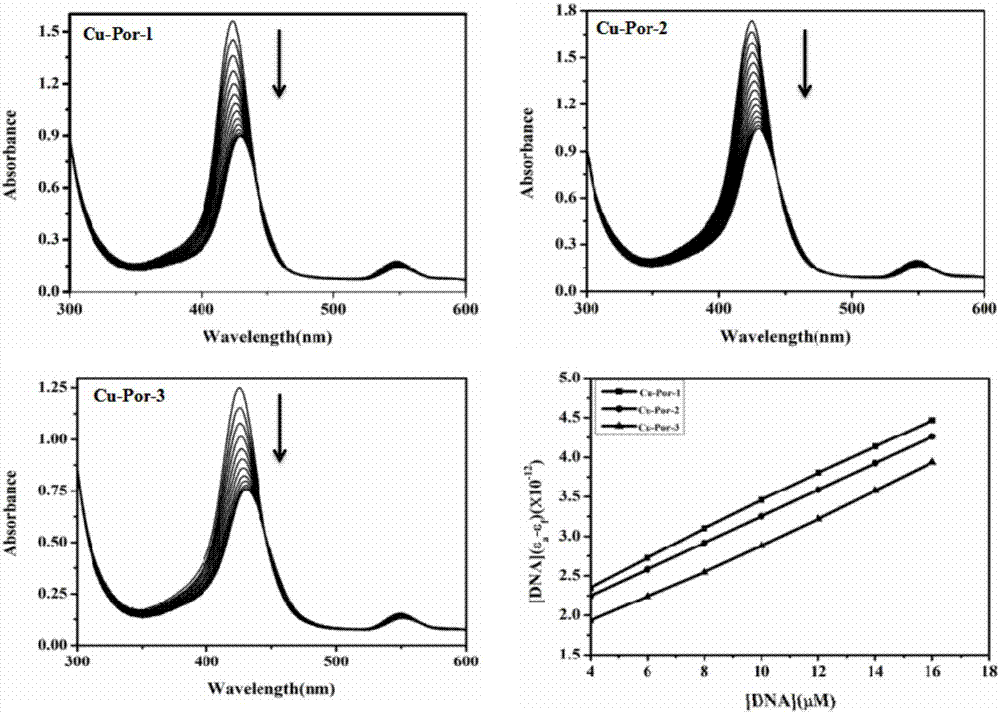
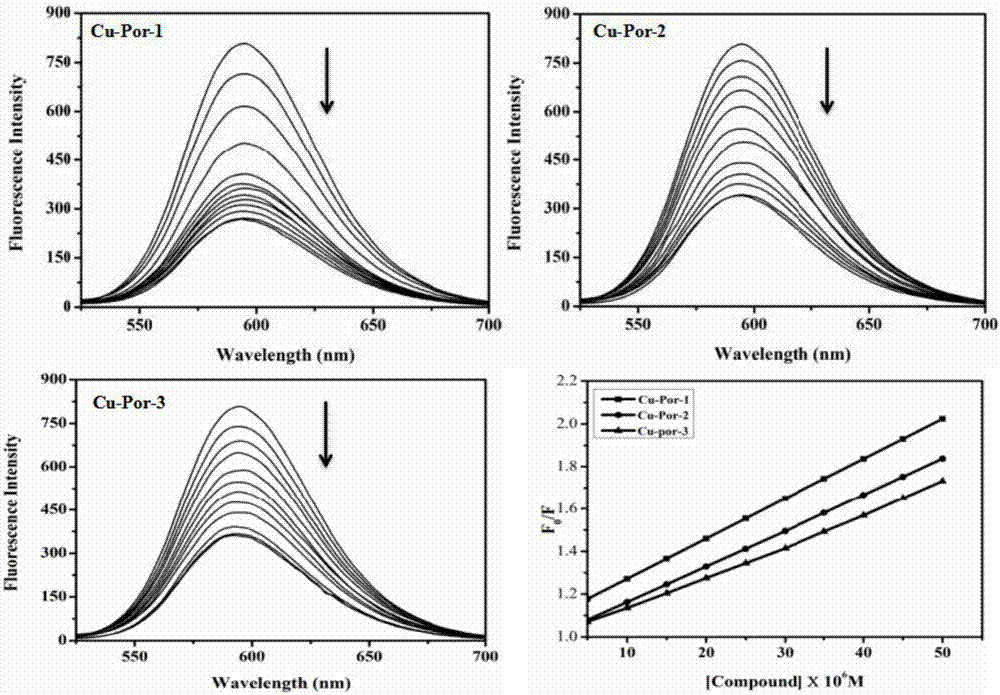
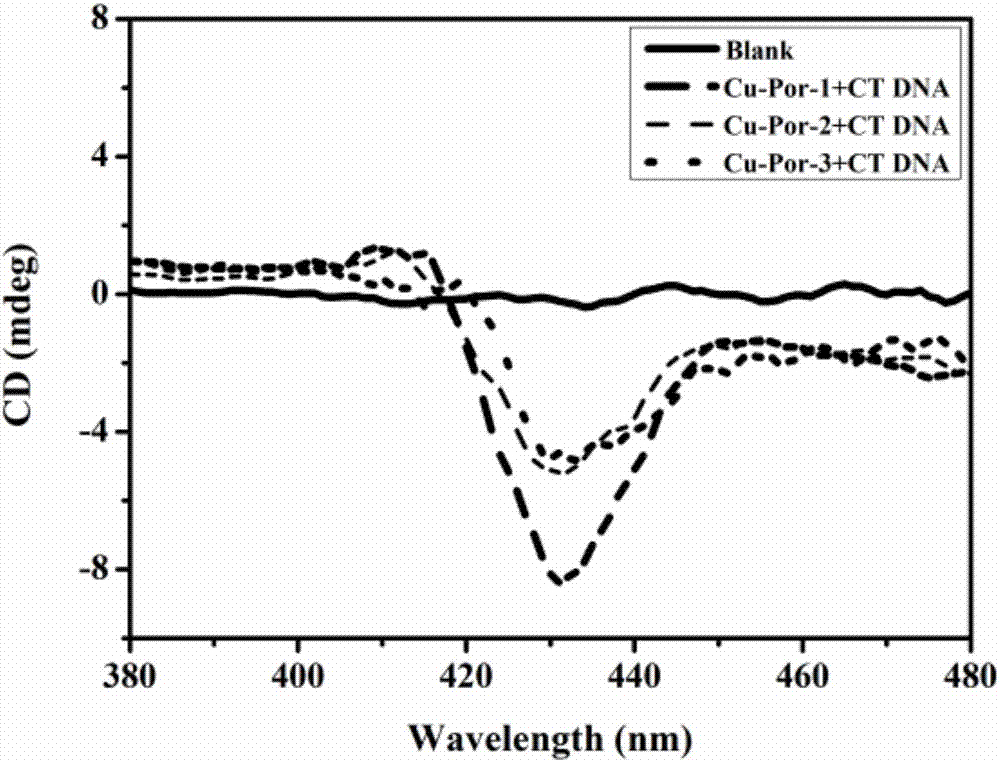
Water-soluble nitryl-containing copper porphyrin and water-soluble Schiff base copper porphyrin complex thereof, as well as synthesis methods and application of water-soluble nitryl-containing copper porphyrin and water-soluble Schiff base copper porphyrin complex thereof
A technology of a porphyrin complex and a synthesis method, applied to the application of water-soluble nitro-containing copper porphyrin in anti-tumor in vitro, a new water-soluble nitro-containing copper porphyrin and a water-soluble Schiff alkali copper porphyrin complex It can solve problems such as limiting the application of porphyrin compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment one, the synthesis of water-soluble nitro-containing copper porphyrin (Cu-Por-1)
[0057] (1) Synthesis of 5,10,15-tri-(4-pyridine)-20-(4-p-nitro)phenyl-porphyrin
[0058] Weigh 1.51g (0.01mol) of p-nitrobenzaldehyde into a 250mL three-necked flask, add a mixed solution of 180mL propionic acid and 15mL propionic anhydride, heat and stir to 130°C, quickly add 2.7mL (0.03mol) 4-pyridinecarbaldehyde, Then slowly add 2.6 mL (0.04 mol) of freshly distilled pyrrole dissolved in 10 mL of propionic acid dropwise with a dropping funnel, complete the addition within 10 min, and reflux at 140°C for 2.5 h. After the reaction, distill under reduced pressure to remove 175mL of solvent and 20ml of remaining solvent, add anhydrous methanol (90ml) 4.5 times the volume of remaining solvent, and stir at room temperature for 0.5h to wash away the pyrrole polymer generated during the reaction. Freeze (-18°C) overnight, and a purple precipitate precipitated out, which was filtere...
Embodiment 2
[0069] Embodiment two, the synthesis of water-soluble Schiff base copper porphyrin complex (Cu-Por-2)
[0070] (1) Synthesis of 5,10,15-three-(4-pyridine)-20-(4-p-nitro)phenyl-porphyrin:
[0071] Same as embodiment one.
[0072] (2) Synthesis of 5,10,15-tri-(4-pyridine)-20-(4-p-amino)phenyl-porphyrin
[0073]Weigh 100mg (0.15mmol) of 5,10,15-tris-(4-pyridine)-20-(4-p-nitro)phenyl-porphyrin in a 100mL three-necked round-bottomed flask, add 60mL (6mol / l) Hydrochloric acid, stir to dissolve. Under the protection of argon, 203 mg (0.9 mmol) of stannous chloride in hydrochloric acid solution was added to the above solution, and reacted at room temperature for 24 hours. After the reaction, neutralize and filter with 5mol / l sodium hydroxide solution, collect the filter cake, dissolve in the mixed solution (V / V=1 / 5) of methanol and dichloromethane, extract with water several times, and collect the organic phase , spin-dried to obtain a purple crude product. The crude product was ...
Embodiment 3
[0088] Embodiment three, the synthesis of water-soluble Schiff base copper porphyrin complex (Cu-Por-3)
[0089] (1) Synthesis of 5,10,15-tris-(4-pyridine)-20-(4-p-nitro)phenyl-porphyrin: same as Example 1.
[0090] (2) Synthesis of 5,10,15-tris-(4-pyridine)-20-(4-p-amino)phenyl-porphyrin: same as Example 2.
[0091] (3) Synthesis of 5,10,15-tri-(4-pyridine)-20-(4-o-hydroxy-m-methoxybenzimino)phenyl-porphyrin
[0092] 100mg (0.15mmol) of 5,10,15-tris-(4-pyridine)-20-(4-p-amino)phenyl-porphyrin was dissolved in 30mL of chloroform, and 115mg (0.75 mmol) o-vanillin in 30mL of methanol solution, then dropwise added 3-5 drops of glacial acetic acid, and refluxed at 72°C for 36h. Glacial acetic acid addition is 0.06~0.1 times of 5,10,15-three-(4-pyridine)-20-(3-methoxy-4-hydroxyl-5-amino)phenyl-porphyrin molar weight, ice Acetic acid acts as a catalyst. After the reaction is completed, vacuum rotary steaming leaves 5 mL of solvent remaining, then dropwise add petroleum ether unt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com