Intermediate for preparing obeticholic acid, preparation method of intermediate and preparation of obeticholic acid
A technology of obeticholic acid and compound, applied in the field of medicine, can solve problems such as unfavorable product quality and cost control, increase of deethylated products, enhanced reaction conditions, etc., to achieve yield improvement, reduction of deethylated by-products, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the compound of formula I obtains the compound of formula II through esterification
[0053]
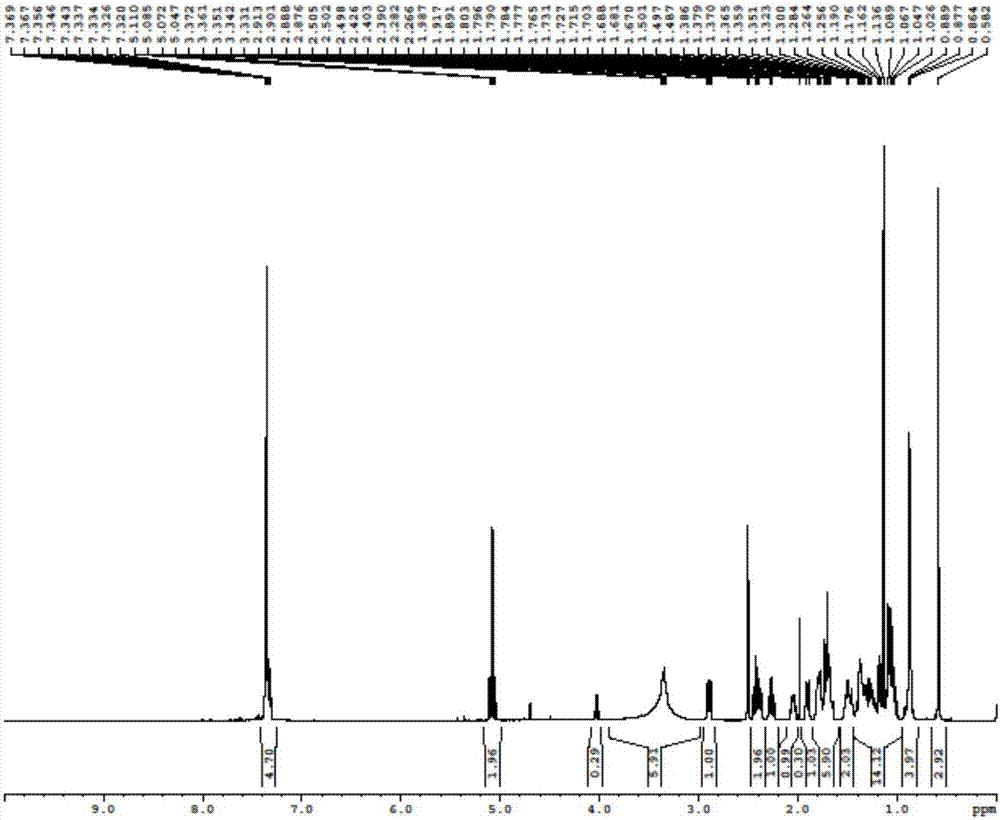
[0054] Add 36g of the compound of formula I into a 500mL reaction flask, add 360mL of acetonitrile, add 45.05g of cesium carbonate while stirring, add 18.91g of benzyl bromide, stir at room temperature overnight, TLC detection, the raw materials are completely reacted. Evaporate acetonitrile under reduced pressure, add 300mL saturated aqueous sodium bicarbonate solution and 200mL ethyl acetate to the concentrated solution, stir to dissolve and separate layers, extract the water layer with 50mL ethyl acetate again, combine the organic layers, wash with 100mL water, and dry over anhydrous sodium sulfate , and concentrated to dryness to obtain 47.4 g of light yellow oil.
[0055] The hydrogen spectrum and mass spectrum data of formula II compound are as follows:
[0056] 1 H NMR (500MHz, DMSO) δ7.72-7.25(m, 15H), 5.08(q, J=12.5Hz, 6H), 4.03(q, J=7.1Hz, 2H), 3.6...
Embodiment 2
[0058] Embodiment 2: the compound of formula II reacts with trimethylchlorosilane to obtain the compound of formula III
[0059]
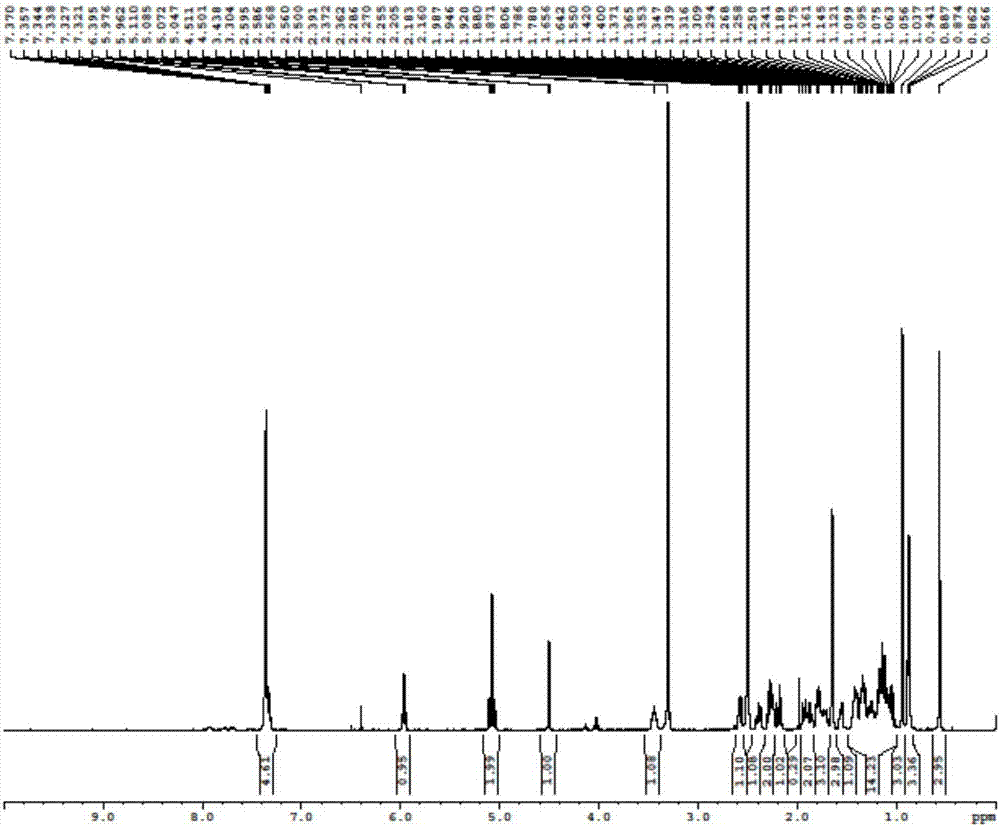
[0060]Add 48.4mL of diisopropylamine and 260mL of tetrahydrofuran to a 1000mL four-necked bottle, replace with nitrogen three times, cool down to -50°C, add 132mL of 2.5M n-butyllithium dropwise, and drop it in about 30 minutes. Control the temperature not to exceed -30°C. After dropping, keep warm at -30°C for 3 hours. Cool down to -65°C, add 34.3mL of trimethylchlorosilane dropwise, and drop it over 10 minutes, control the temperature not to exceed -50°C, keep it warm for 30 minutes after dropping, add 13.2g of the compound of formula (II) in 100mL of tetrahydrofuran solution dropwise, drop for about 20 minutes After completion of dripping, keep at -50°C for 1h. TLC detection, the raw material reacted completely. Naturally raise the temperature to -40°C, add 68.6mL triethylamine, raise the temperature to -20°C, add 200mL saturated sodium bic...
Embodiment 3
[0063] Embodiment 3: compound of formula III and acetaldehyde obtain formula IV compound through aldol condensation
[0064]
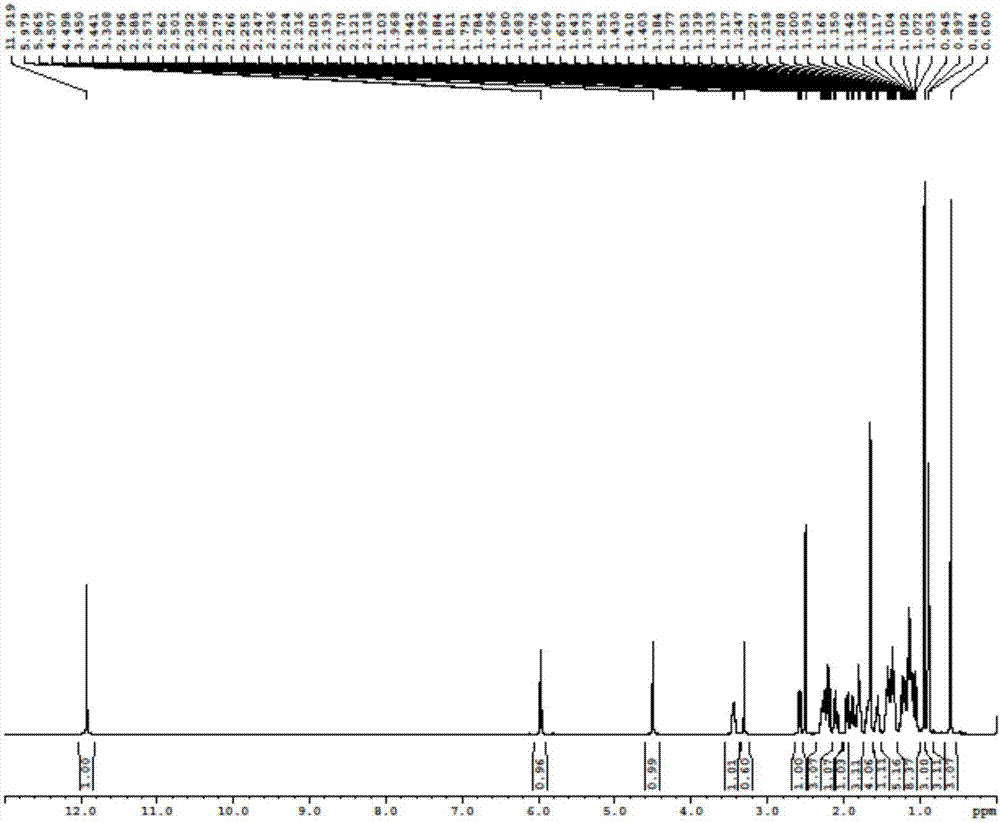
[0065] Add 23.6g of the compound of formula (III) in 300mL of dichloromethane solution into a 500mL four-neck flask, replace with nitrogen three times, cool down to -65°C, add 8.6mL of acetaldehyde, dropwise add 48.5mL of boron trifluoride diethyl ether, and drop it in 10 minutes. Control the temperature not to exceed -60°C, keep stirring for 4 hours, then naturally warm up to room temperature, stir overnight, and TLC detection shows that the raw materials have completely reacted. Slowly add 500mL of saturated sodium bicarbonate, a large number of bubbles emerge, separate layers, extract the aqueous layer with 200mL of dichloromethane, combine the organic layer, wash with 200mL of saturated sodium chloride, dry over anhydrous sodium sulfate, decolorize with activated carbon, and concentrate under reduced pressure. 20.6 g of a reddish-brown oil were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com