Magnetic resonance contrast agent using human endogenous protein as chelating agent and preparation thereof
A technology of magnetic resonance contrast agent and endogenous protein, which is applied in nuclear magnetic resonance/magnetic resonance imaging contrast agent, preparations for in vivo experiments, pharmaceutical formulations, etc. Poor scalability, narrow imaging time window, etc., to achieve the effect of easy mass preparation, good biocompatibility and relaxation stability, and long blood half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
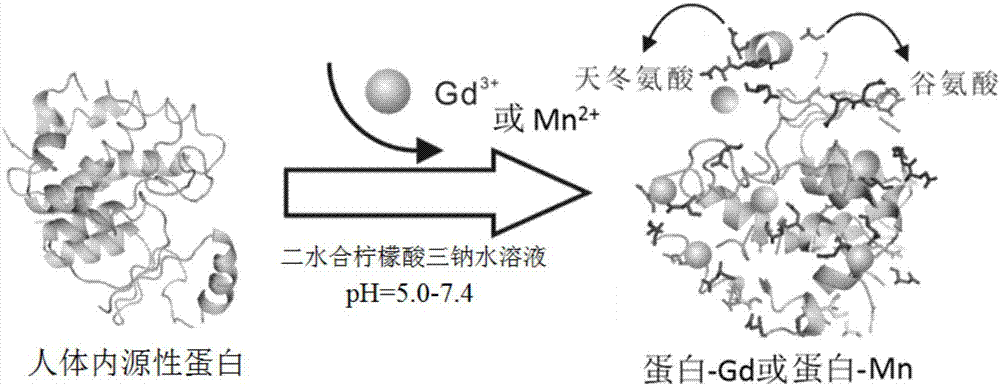
[0036] like figure 1 As shown, the preparation method of a magnetic resonance contrast agent using human endogenous protein as a chelating agent comprises the following steps:
[0037] 1) Weigh 290 mg (about 0.01 mmol) of glutathione transferase (GST) powder, dissolve it in 2.0 mL of ultrapure water, and stir on a 37°C water bath stirrer;
[0038] 2) Add 2.0 mL of 50 mg / mL trisodium citrate dihydrate aqueous solution into the above GST protein solution, the final concentration of trisodium citrate dihydrate aqueous solution is 25 mg / mL, and continue stirring;
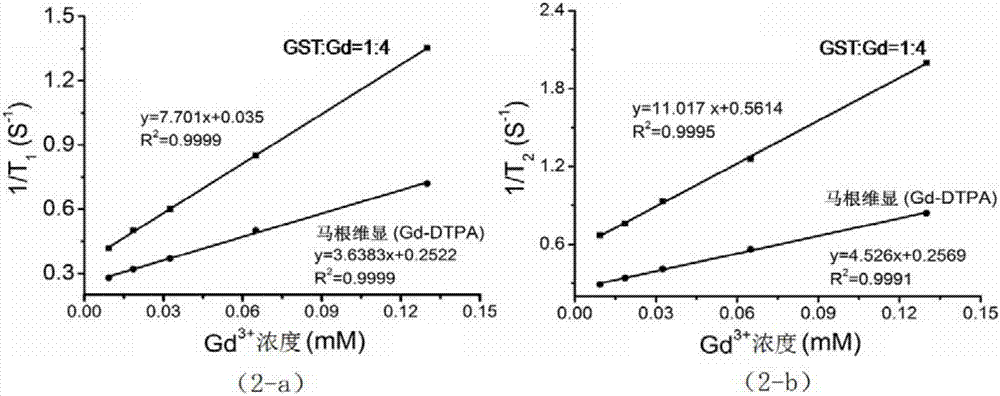
[0039] 3) Measure the aqueous solution of gadolinium nitrate according to the molar ratio of protein and paramagnetic metal ion being 1:4, add it dropwise to the mixed solution in 2), continue to stir and react, carry out paramagnetic ion chelation, and obtain the reaction solution;
[0040] 4) Use acetic acid-sodium acetate buffer solution to adjust the pH value of the reaction solution to 5.0;
[0041] 5) After the re...
Embodiment 2
[0045] like figure 1 As shown, the preparation method of a magnetic resonance contrast agent using human endogenous protein as a chelating agent comprises the following steps:
[0046] 1) Weigh 435 mg (about 0.015 mmol) of glutathione transferase (GST) powder, dissolve it in 3.0 mL of ultrapure water, and stir on a water bath stirrer at 37°C;
[0047] 2) Add 2.0 mL of 50 mg / mL trisodium citrate dihydrate aqueous solution to the above GST protein solution, the final concentration of trisodium citrate dihydrate aqueous solution is 20 mg / mL, and continue stirring;
[0048] 3) Measure the aqueous solution of gadolinium chloride according to the molar ratio of protein to paramagnetic metal ion being 1:8, add it dropwise to the mixed solution in 2), continue to stir and react, and perform paramagnetic ion chelation to obtain a reaction solution;
[0049] 4) Use acetic acid-sodium acetate buffer solution to adjust the pH value of the final reaction solution to 6.5;
[0050] 5) Afte...
Embodiment 3
[0055] like figure 1 As shown, the preparation method of a magnetic resonance contrast agent using human endogenous protein as a chelating agent comprises the following steps:
[0056] 1) Weigh 580 mg (about 0.02 mmol) of glutathione transferase (GST) powder, dissolve it in 4.0 mL of ultrapure water, and stir on a 37°C water bath stirrer;
[0057] 2) Add 1.0 mL of 50 mg / mL trisodium citrate dihydrate aqueous solution into the above GST protein solution, the final concentration of trisodium citrate dihydrate aqueous solution is 10 mg / mL, and continue stirring;
[0058] 3) Measure the aqueous solution of gadolinium acetate according to the molar ratio of protein and paramagnetic metal ion of 1:16, add it dropwise to the mixed solution in 2), continue to stir and react, and perform paramagnetic ion chelation to obtain a reaction solution;
[0059] 4) Use acetic acid-sodium acetate buffer solution to adjust the pH value of the final reaction solution to 7.0;
[0060] 5) After th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com