A kind of nimodipine solid dispersion and tablet preparation method thereof
A nimodipine solid and solid dispersion technology, which is applied in the field of medicine, can solve problems such as recrystallization and crystallization, and achieve the effects of accelerated dissolution, low hygroscopicity, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
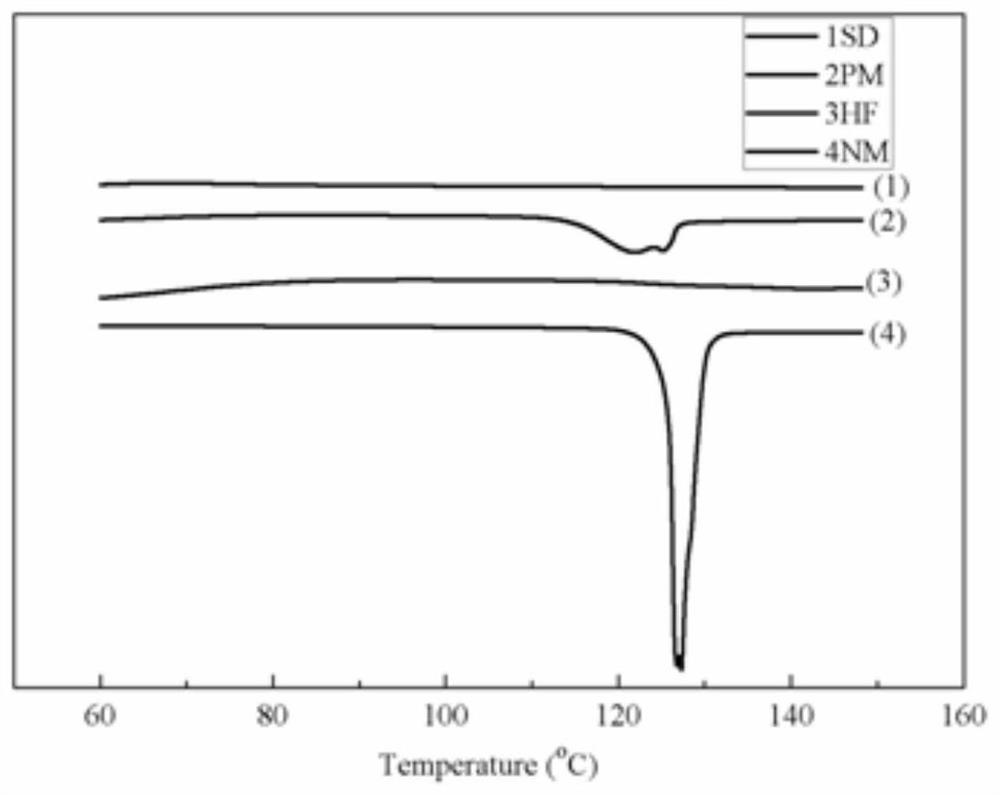
[0035] Example 1 Differential scanning calorimetry analysis: Weigh 4mg of the sample and place it in an aluminum pan, using alumina as a reference, heat up in a nitrogen flow in the range of 25°C to 150°C at a rate of 10°C / min scanning.
[0036] Such as figure 1 As shown, it can be seen that the melting point of the nimodipine bulk drug (NM) is 125°C-127°C, and the bulk drug is transformed from a crystalline state to an amorphous state after being prepared into a solid dispersion (SD).
Embodiment 2
[0038](1) Prepare 5000 mL of phosphate buffer solution of pH 6.8;
[0039] (2) Prepare nimodipine solution: take by weighing 300 mg of nimodipine crude drug into a 10 mL measuring bottle, add absolute ethanol to dissolve and settle to the mark;
[0040] (3) Weigh PVP K30 70.2g, PVP K25 71.1g, HPMCAS-HF 70.3g, HPMCAS-HF120.9g, HPMCAS-HF 271.5g into a beaker, add 200mL of pH6.8 phosphate buffer solution, ultrasonically It dissolves, moves in the dissolution cup, adds the phosphate buffer solution of remaining 700mL pH6.8, adjusts the speed of dissolution apparatus to be 75rpm, and temperature is 37 ℃, respectively gets 1mL above-mentioned nimodipine solution of preparation in each dissolution cup, Samples were taken at different time points to detect the content of nimodipine.
[0041] The experimental results are shown in Table 1:
[0042] Table 1
[0043]
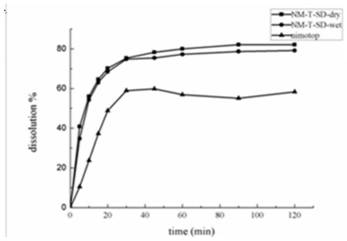
[0044] Such as figure 2 And the above results show that HPMCAS-HF has a better crystallization inhibitory effect ...
Embodiment 3
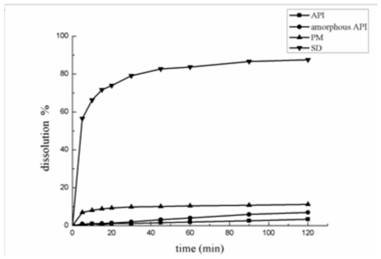
[0045] Example 3 Accurately weigh 30.0 mg of nimodipine bulk drug onto weighing paper, place it in a blast drying oven heated to 130°C for 30 minutes, take it out and put it at room temperature to obtain amorphous nimodipine bulk drug , taking nimodipine crystalline API (API), amorphous API (amorphous API), 30% NM-HPMCASHF physical mixture (PM), solid dispersion (SD) for in vitro dissolution experiments.
[0046] Such as image 3 As shown, it can be seen that the dissolution rate of nimodipine and HPMCASHF auxiliary materials through the solid dispersion (SD) prepared by hot-melt extrusion technology is the highest.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com