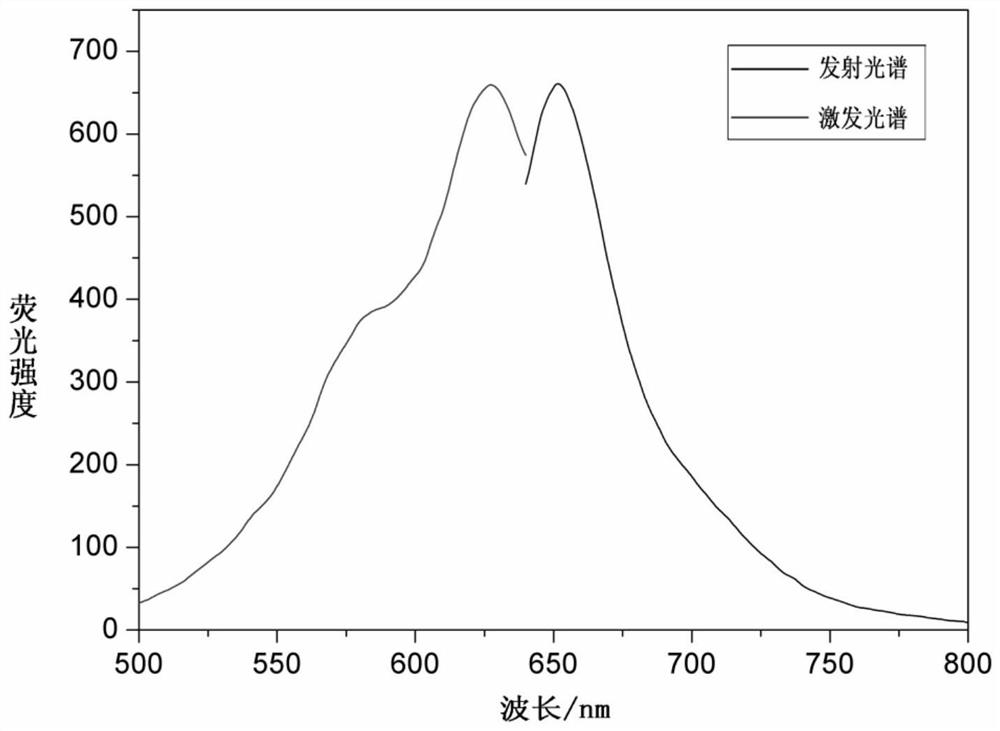
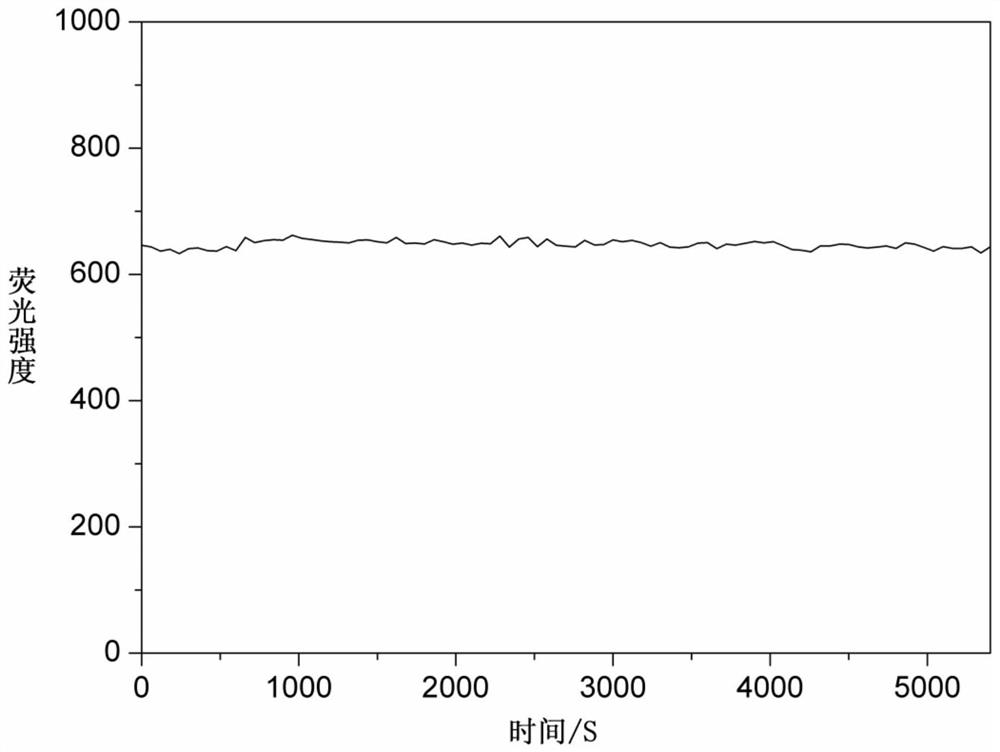
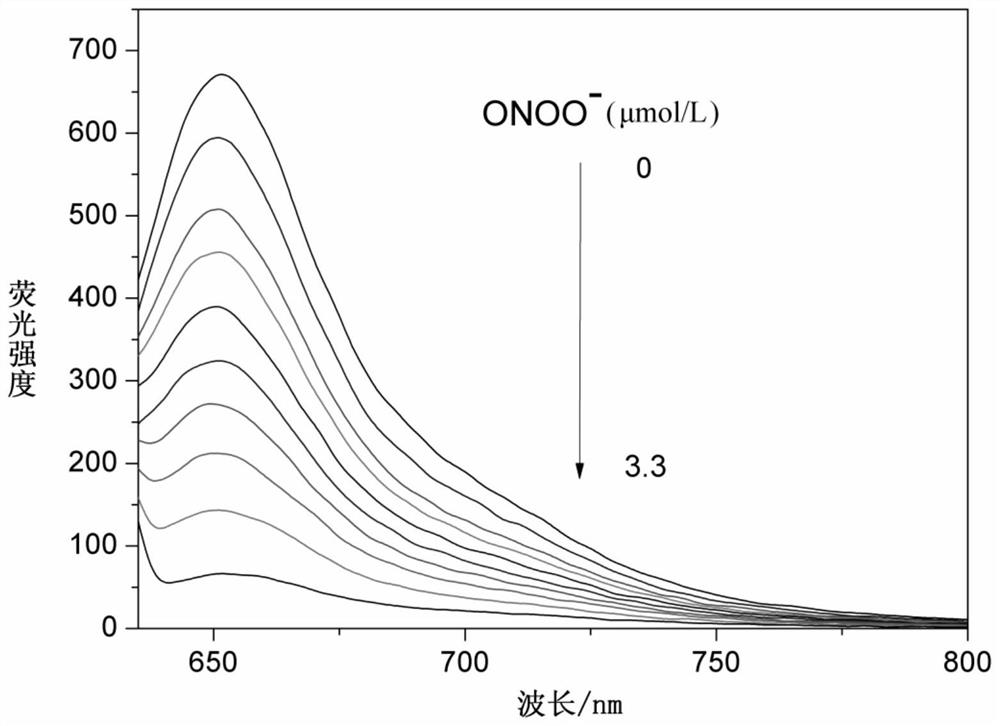
A cyanine-based visible organic molecular fluorescent probe and its preparation method
A fluorescent probe and organic molecule technology, applied in the field of fluorescence spectrum detection and analysis, can solve the problems of specificity and sensitivity to be improved, long reaction time, etc., and achieve the effects of good probe stability, short action time and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1. Preparation of organic molecular fluorescent probes, the structural formula is as follows:
[0047]
[0048] (1) In a 50mL round bottom flask, 0.147mol phosphorus oxychloride (POCl 3 ) was added dropwise to 0.518mol of N,N-dimethylformamide (DMF, C 3 h 7 NO), keep the temperature below 5°C, remove the ice bath, and stir the mixture at room temperature for 1 hour; add 0.051mol bromoacetic acid, and heat the mixture at 70°C for 24 hours. After the reaction, add ice water to terminate the reaction and extract and remove the DMF dissolved in water, add a large amount of sodium carbonate until the pH=8, add absolute ethanol to filter out the inorganic salt, evaporate the obtained organic filtrate on a rotary evaporator until the solvent is completely removed, With 10mL, 50% H 2 SO 4 Neutralize the pale yellow residue with 200 mL CH 3 Cl extraction 3 times with MgSO 4 After drying and removing the solvent, triformylmethane is obtained, namely compound II;
[0049...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com