Stable rhEndostatin subcutaneous injection composition
A vascular endothelium and statin technology, which can be used in drug combinations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Stability, pain-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The screening of embodiment 1 buffer system
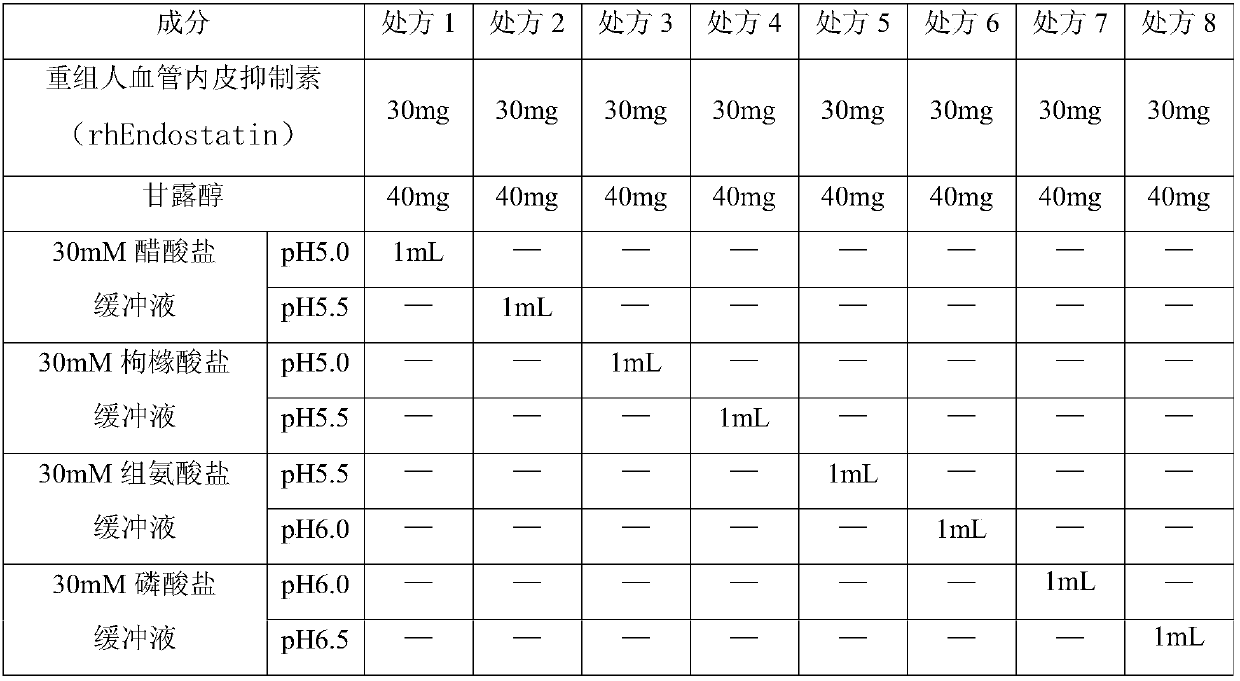
[0027] (1) Prescription preparation
[0028] The pH value of the human body is 7.4, and the pH value of the injection is generally required to be between 4 and 9. In addition, in order to ensure that the quality of the finished product is stable during the storage period, it is necessary to determine the reasonable pH range of the medicinal solution. The inventors prepared the solutions according to Table 1, and sealed them in vials for storage. Wherein, in the buffer system , the acetate buffer is acetic acid-sodium acetate buffer, the citrate buffer is citric acid-sodium citrate buffer, and the histidine buffer is histidine-histidine hydrochloride buffer, Phosphate buffer is sodium dihydrogen phosphate-disodium hydrogen phosphate buffer.
[0029] Table 1 List of prescriptions
[0030]
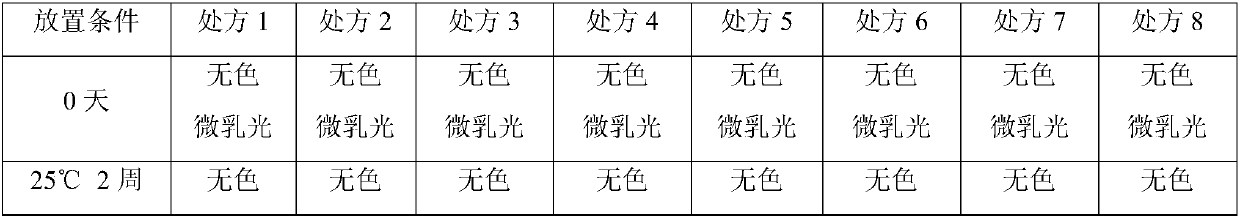
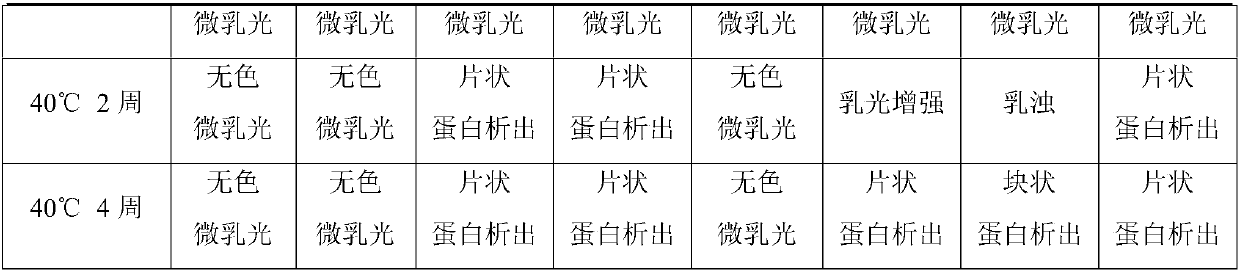
[0031] (2) Accelerated test
[0032] The appearance and purity of the above prescriptions were checked by using accelerated experim...
Embodiment 2
[0043] The influence of embodiment 2 stabilizer sugars and surfactant
[0044] (1) Prescription preparation
[0045] Select acetate buffer system and histidine salt buffer system to study the influence of carbohydrates and surfactants on the stability of recombinant human vascular endostatin (rhEndostatin). Store in bottle. Wherein, in the buffer system, the acetate buffer is acetic acid-sodium acetate buffer, the histidine buffer is histidine-histidine hydrochloride buffer, and the phosphate buffer is sodium dihydrogen phosphate- Disodium hydrogen phosphate buffer.
[0046] Table 4 Prescription List
[0047]
[0048]
[0049] (2) Accelerated test
[0050] The appearance and purity of the above prescriptions were checked by accelerated experiments, which were stored at 25°C for 2 months, at 25°C for 6 months, at 40°C for 2 weeks, and at 40°C for 4 weeks.
[0051] When checking the purity, use HPLC to measure the rhEndostatin purity, and the chromatographic column of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com