Preparation method of polyethylene glycol grafted poly-n-butyl methacrylate amphiphilic grafted copolymer
A polybutyl methacrylate and polyethylene glycol grafting technology, applied in the field of chemical materials, can solve the problem of less research on amphiphilic graft polymers, and achieve controllable molecular weight of side chains and adjustable graft density. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
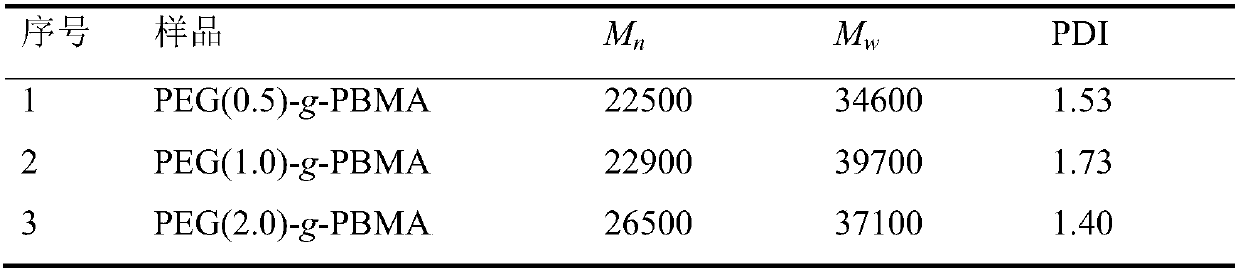
Examples
Embodiment 1
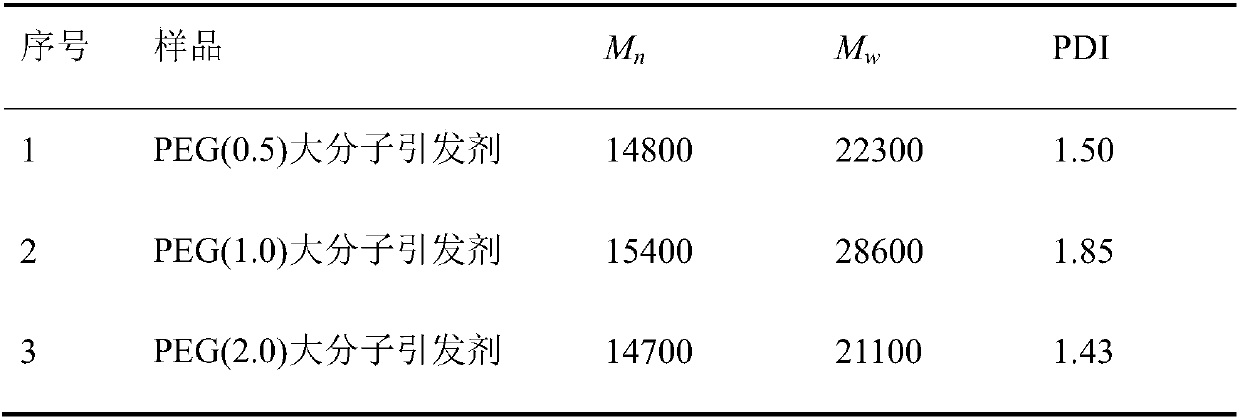
[0027] (1) Preparation of small molecule dihydroxy initiator
[0028] Add 800mL of solvent tetrahydrofuran, 74.6g (0.50mol) of triethanolamine, and 5.06g (0.05mol) of acid-binding agent triethylamine into a four-neck flask equipped with a thermometer and a stirring paddle, place in an ice-water bath, stir mechanically, and wait for the system to When the internal temperature dropped below 3°C, 11.50 g (0.05 mol) of α-bromoisobutyryl bromide was added as slowly as possible through a constant-pressure dropping funnel. After the addition was complete, the ice-water bath was removed, and the reaction was carried out overnight at room temperature. After the reaction was completed, the generated salt was removed by filter paper filtration, the filtrate was evaporated under reduced pressure to remove the solvent tetrahydrofuran, the concentrated solution was dissolved in ethyl acetate, passed through an alkaline alumina column, collected and evaporated to obtain the product. After dr...
Embodiment 2
[0035] (1) Preparation of small molecule initiator
[0036]Add 800mL of solvent tetrahydrofuran, 74.6g (0.50mol) of triethanolamine, and 5.06g (0.05mol) of acid-binding agent triethylamine into a four-neck flask equipped with a thermometer and a stirring paddle, place in an ice-water bath, stir mechanically, and wait for the system to When the internal temperature dropped below 3°C, 10.79 g (0.05 mol) of α-bromopropionyl bromide was added as slowly as possible through a constant-pressure dropping funnel. After the addition was complete, the ice-water bath was removed, and the reaction was carried out overnight at room temperature. After the reaction was completed, the generated salt was removed by filter paper filtration, the filtrate was evaporated under reduced pressure to remove the solvent tetrahydrofuran, the concentrated solution was dissolved in ethyl acetate, passed through an alkaline alumina column, collected and evaporated to obtain the product. After drying in a va...
Embodiment 3
[0043] (1) Preparation of small molecule initiator
[0044] Add 800 mL of tetrahydrofuran as a solvent, 67.09 g (0.50 mol) of trimethylolpropane, and 5.06 g (0.05 mol) of acid-binding agent triethylamine into a four-neck flask equipped with a thermometer and a stirring paddle, place in an ice-water bath, and stir mechanically , when the internal temperature of the system dropped below 3°C, 11.50 g (0.05 mol) of α-bromoisobutyryl bromide was added as slowly as possible through a constant pressure dropping funnel. After the addition was complete, the ice-water bath was removed and the reaction was carried out overnight at room temperature. After the reaction was completed, the generated salt was removed by filter paper filtration, the filtrate was evaporated under reduced pressure to remove the solvent tetrahydrofuran, the concentrated solution was dissolved in ethyl acetate, passed through an alkaline alumina column, collected and evaporated to obtain the product. After drying ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com