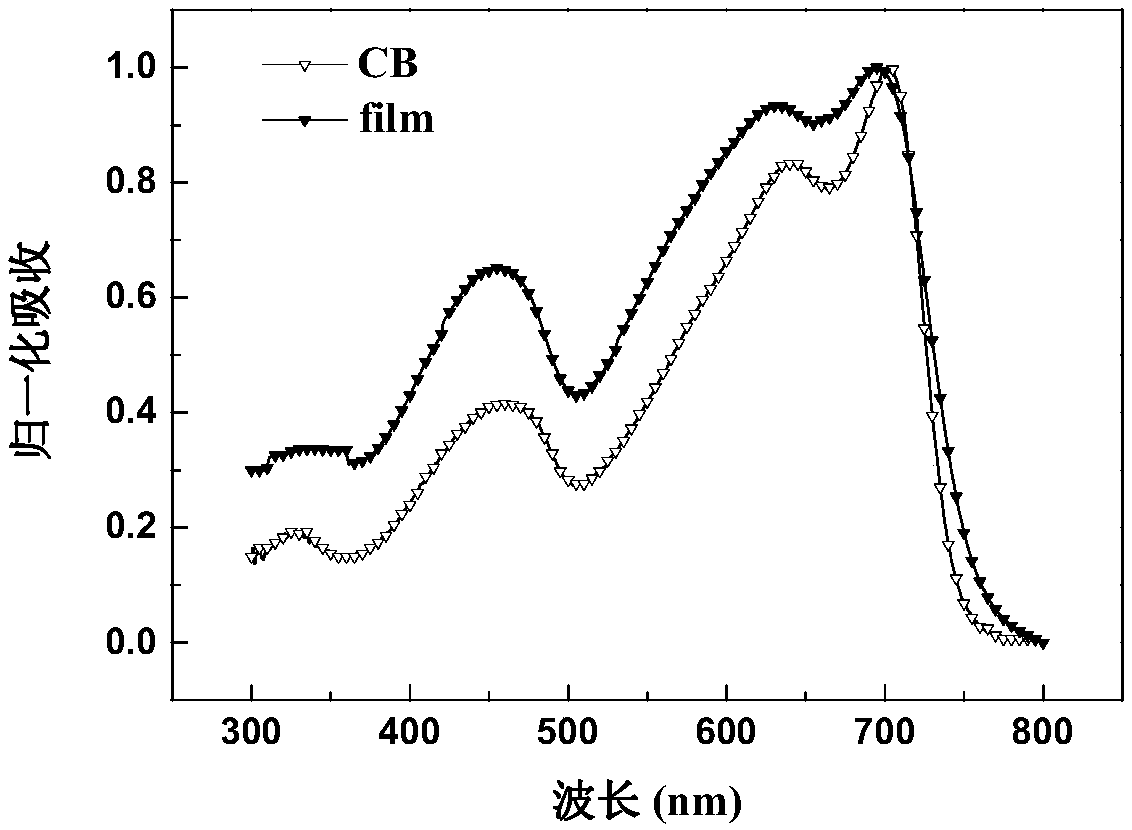
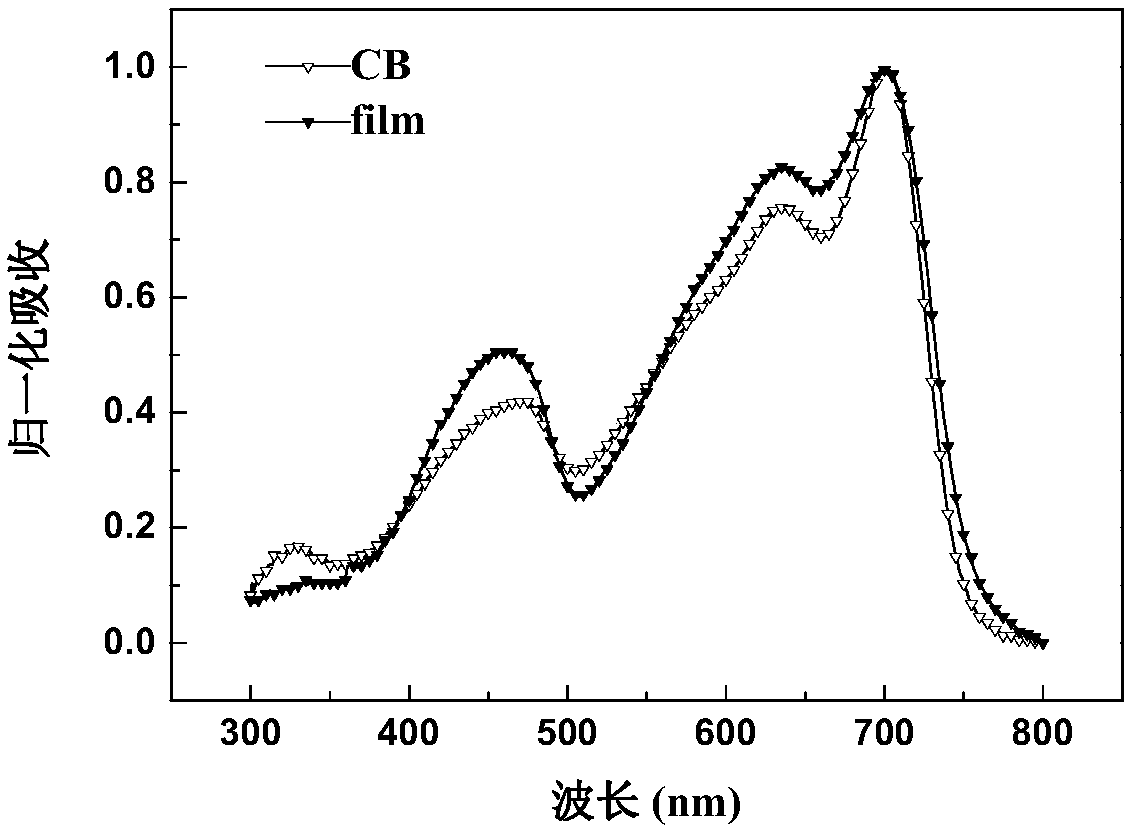
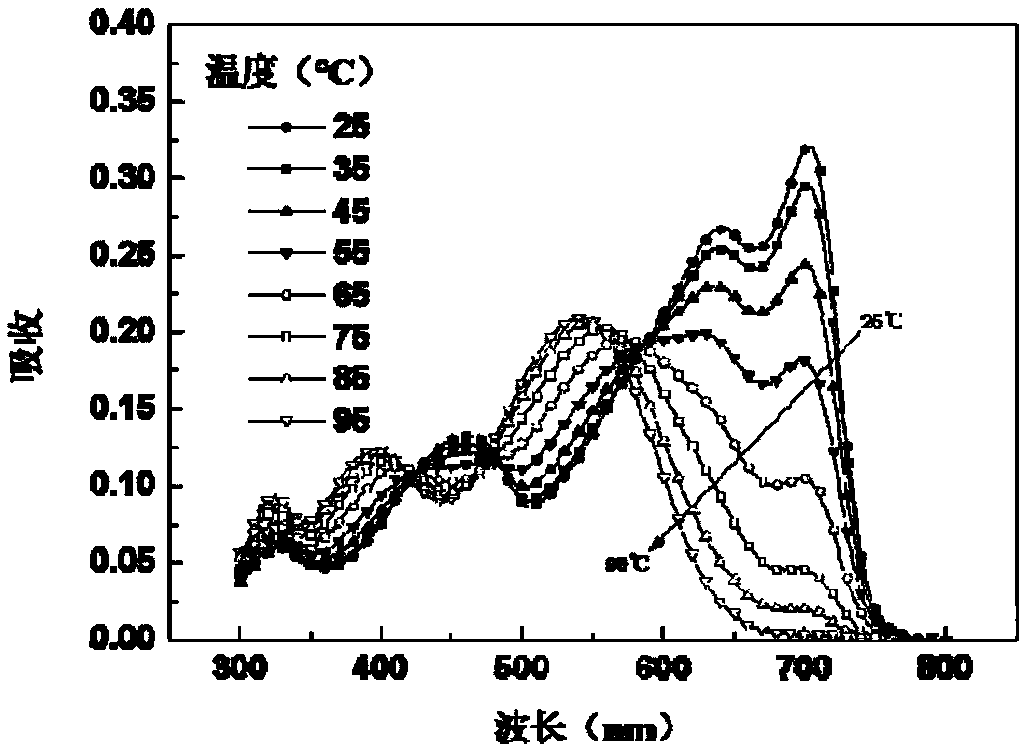
Random copolymer with difluorobenzothiadiazole and quaterthiophene as main chain as well as preparation method and application of copolymer
A technology of difluorobenzothiadiazole and benzothiadiazole, which is applied in the field of random copolymers and its preparation and application, and can solve problems such as film production that have not been met yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of 5,6 difluoro-4,7-dibromo-2,1,3-benzothiadiazole, the reaction formula is as follows:
[0023]
[0024] Add 5,6 difluoro-2,1,3-benzothiadiazole (3.44g, 20mmol) into a 250ml flask, add 100ml of concentrated sulfuric acid, after fully dissolving, add DBDMH (dibromohydantoin) in three batches ) (6.86g, 24mmol), stirred at 70°C for 4 hours. The reactant was poured into ice water, and the crude product was obtained by suction filtration, which was recrystallized with ethanol after passing through the column, and a white solid was obtained after suction filtration. through 13 CNMR analysis test showed that it was the target product 5,6-difluoro-4,7-dibromo-2,1,3-benzothiadiazole.
[0025] 13 C NMR (300MHz, CDCl3) δ (ppm) 153.67, 153.39, 150.20, 149.92, 148.84, 99.51, 99.39, 99.20.
Embodiment 2
[0027] The preparation of 4,7-bis[4-(2-decyltetradecyl)thiophen-2-yl]-5,6-difluoro-2,1,3-benzothiadiazole has the following reaction formula:
[0028]
[0029] Add 5,6 difluoro-4,7-dibromo-2,1,3-benzothiadiazole (330mg, 1.00mmol), 2-(tributyltin)-4-(2 -decyltetradecyl)thiophene (2.12g, 3.00mmol), nitrogen gas was passed for 30 minutes, then 268 mg of bis(triphenylphosphine)palladium dichloride was added, 15 ml of anhydrous toluene was added under nitrogen protection, and heated The reaction was refluxed for two days. Cool to room temperature after the reaction, pour into 100 ml of water, extract with dichloromethane and dry the organic phase with anhydrous magnesium sulfate, remove the solvent after separation, and separate with a silica gel column to obtain a yellow floc. through 1 HNMR, 13 CNMR analysis showed that the target product was 4,7-bis[4-(2-decyltetradecyl)thiophen-2-yl]-5,6-difluoro-2,1,3-benzothiadiazole .
[0030] 1 H NMR (300MHz, CDCl3) δ (ppm) 8.09 (d...
Embodiment 3
[0033] Preparation of 4,7-bis[5-bromo-4-(2-decyltetradecyl)thiophen-2-yl]-5,6-difluoro-2,1,3-benzothiadiazole, The reaction formula is as follows:
[0034]
[0035] Add 4,7-bis[4-(2-decyltetradecyl)thiophen-2-yl]-5,6-difluoro-2,1,3-benzothiadi Azole (808mg, 0.8mmol) and 15ml of tetrahydrofuran were added with bromosuccinimide (NBS) (315mg, 1.76mmol) under full stirring, and reacted in the dark at room temperature for 24 hours. After the reaction, add the reaction solution into water, extract with dichloromethane and wash the organic phase with water, dry the organic phase with anhydrous sodium sulfate, separate, remove the solvent and separate with a silica gel column to obtain an orange-red solid. through 1 HNMR, 13 CNMR, and elemental analysis tests showed that it was the target product 4,7-bis[4-(2-decyltetradecyl)thiophen-2-yl]-5,6-difluoro-2,1,3-benzo Thiadiazoles.
[0036] 1H NMR (300MHz, CDCl3) δ (ppm) 7.89 (s, 2H), 2.56 (d, J = 7.0Hz, 4H), 1.73 (s, 2H), 1.27 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com