A method for producing fluoroberyllic acid and ammonium fluoroberyllium from beryllium sulfate solution
A technology of beryllium sulfate and fluoroberyllic acid, which is applied in the direction of improving process efficiency, etc., can solve the problems of low direct yield and total yield in the production process, affecting the quality of beryllium beads, and removing impurities, etc., so as to improve the recovery rate, The effect of reducing production cost and stabilizing quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
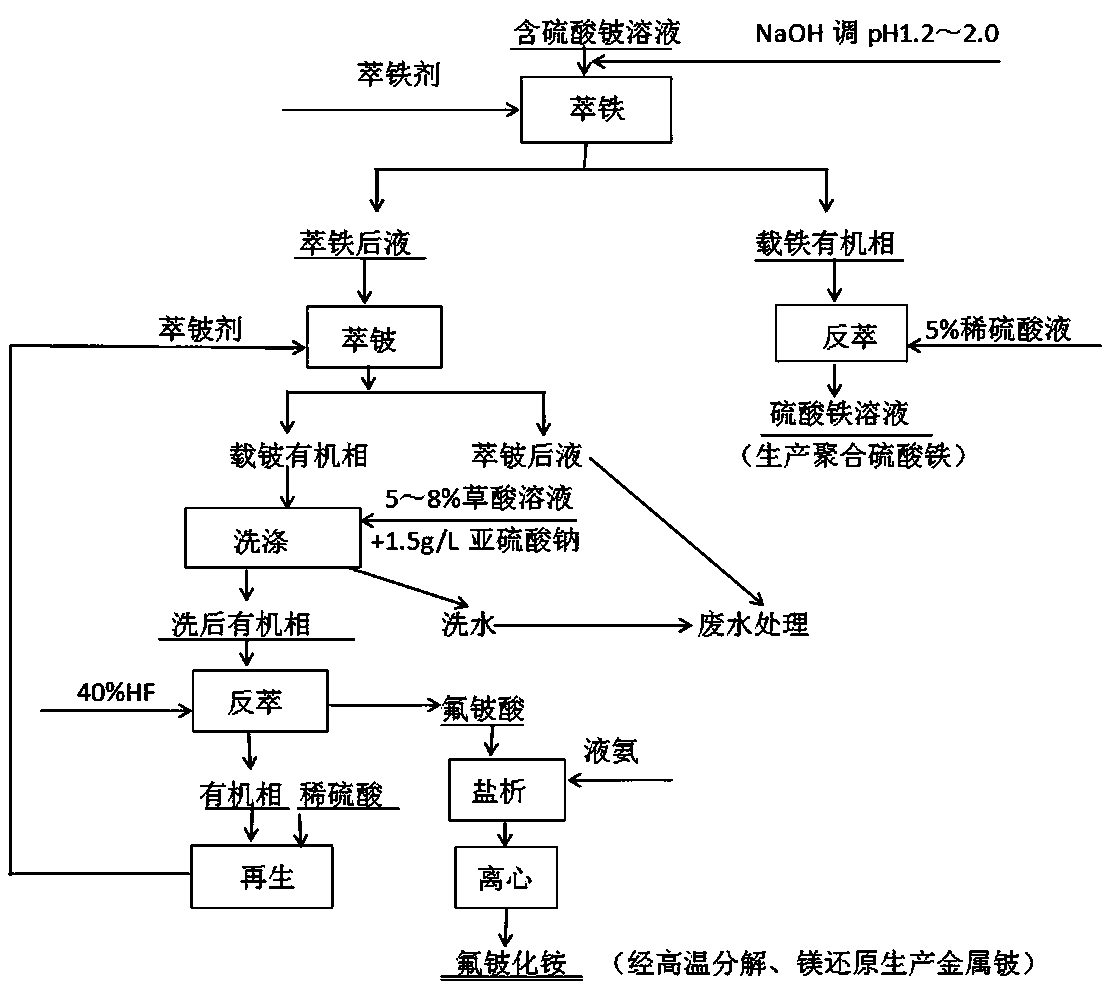
[0034] This example provides a production method for extracting beryllium compounds and beryllium from a solution containing beryllium sulfate. The main components of the solution containing beryllium sulfate are (g / L) Be 5.43, Fe 3.76, Al 14.55, and Si 0.09. Include the following steps:
[0035] (1) Iron extraction
[0036] Use NaOH to adjust the pH value of the sulfuric acid solution to 1.2-2.0 as the iron extraction stock solution. The iron extraction agent is composed of 40-50% N-235 + 10-25% 2-octanol + 25-50% kerosene by volume percentage. The newly prepared iron extraction agent needs to be treated with 2N hydrochloric acid solution. Compared with O:A (organic phase: aqueous phase) = 1:1, the series is 5, contact time: stay in the mixing chamber for 5-10 minutes, the effect of iron removal can be 10% ammonium thiocyanate (or thiocyanate Potassium) to identify the effluent water phase until it is colorless or reddish. The iron stripping agent is 5% sulfuric acid solu...
Embodiment 2
[0046] The main components of the solution containing beryllium sulfate in this example are (g / L) Be 2.8, Fe 4.11, Al 12.75, and Si 1.05. The treatment method is the same as that of Example 1, the only difference is that the ratio of extracting beryllium in step (2) is adjusted to O / A=1.5:1, and the rest of the steps are the same.
Embodiment 3
[0048] The main components of the solution containing beryllium sulfate in this example are (g / L) Be 1.95, Fe 3.58, Al 13.8, Si1.12. The treatment method is the same as that in Example 1, except that the ratio of beryllium extraction in step (2) is adjusted to O / A=1:1, and the rest of the steps are the same.
[0049] The composition of the ammonium fluoroberyllium obtained according to Examples 1-3 is as follows: Be>7.0%, Fe<0.0045%, Al<0.003%, Si<0.001%, which can meet the quality requirements for producing high-purity beryllium beads . The recovery rate is increased by 8-10% compared with the existing technology.
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com