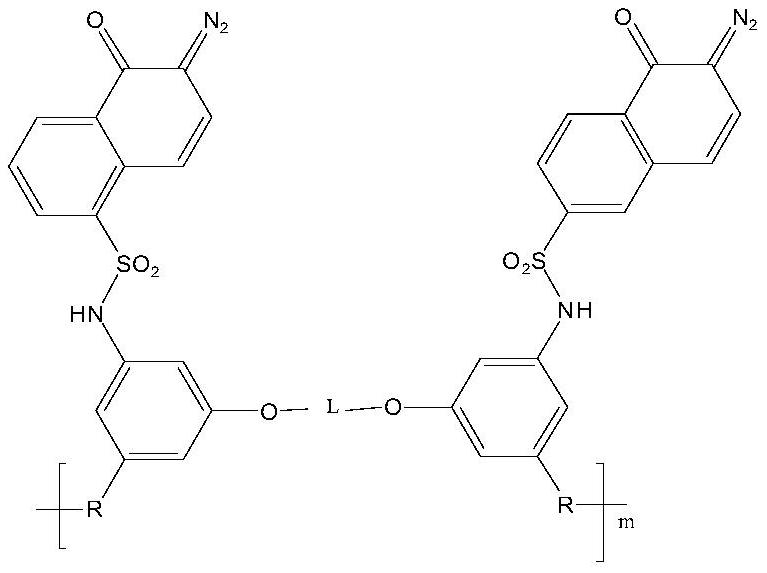
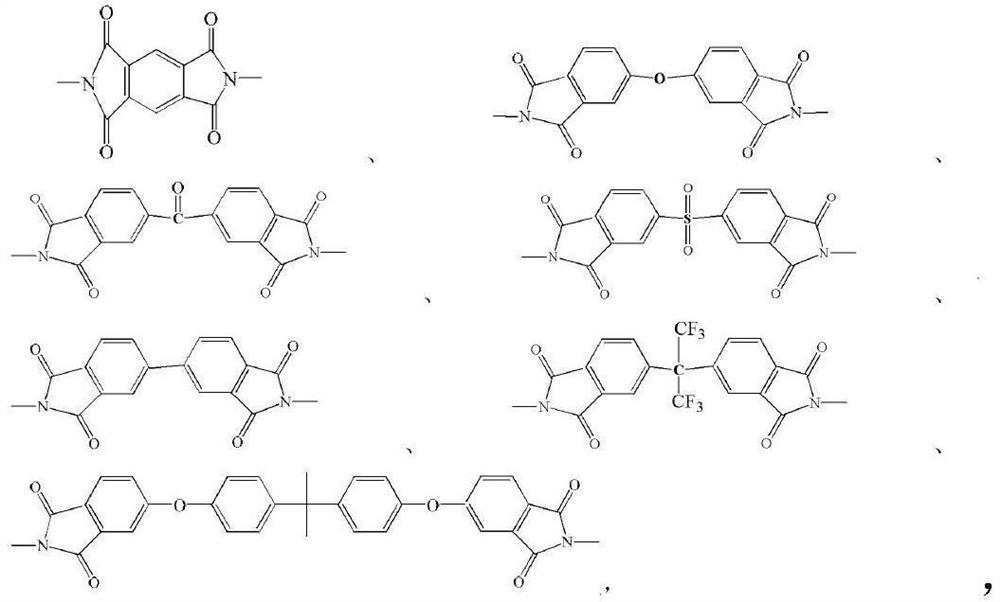
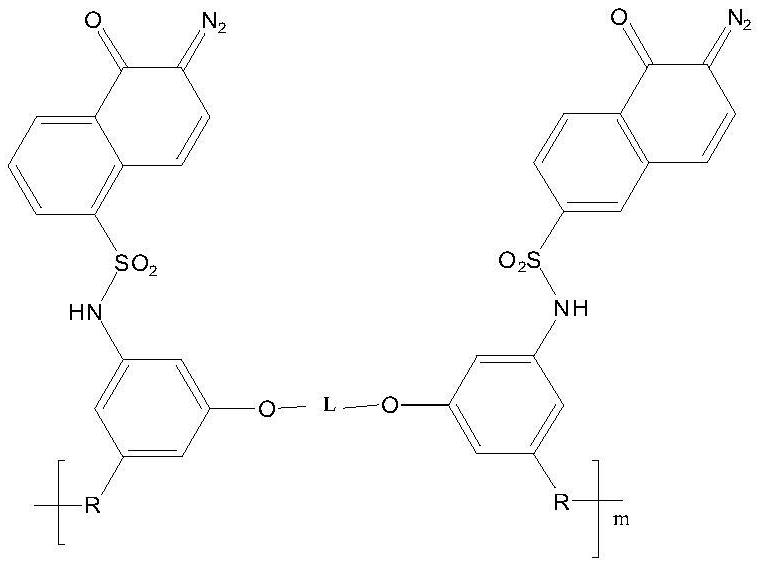
A kind of photosensitive polyimide and preparation method thereof
A photosensitive polyimide and polyimide technology, applied in the field of photosensitive polyimide and its preparation, can solve the problems of poor resolution, increased solubility difference of polyamic acid, large acting force, etc. High, good photosensitivity, good film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] a. Dissolve α, ω-bis(methylamino)polydimethylsiloxane (0.02mol) halogenated diaminophenoxyphenyl hexafluoropropane (0.045mol) in ethanol, add triethylamine After reacting for 5 hours, the substituted product was separated by distillation under reduced pressure;
[0033] b. Dissolve the above-mentioned substituted product in N, N'-dimethylformamide, the solute mass percentage concentration is 15%, after completely dissolving, cool to room temperature, add dianhydride (0.045mol), nitrogen protection, at 10°C Next, stir and react for 3 hours to obtain a polyamic acid solution;
[0034] c. Add p-hydroxyaniline (0.00025mol), and react at room temperature for 2 hours to obtain a polyamic acid solution terminated with phenolic hydroxyl groups;
[0035] d. adding acetic anhydride and pyridine for dehydration imidization reaction, reacting at 50°C for 7 hours, after the reaction is completed, settling, filtering, washing, and vacuum drying to obtain polyimide powder terminated ...
Embodiment 2
[0040] a. Dissolve α, ω-bis(methylamino)polydimethylsiloxane (0.03mol) halogenated diaminophenoxyphenyl hexafluoropropane (0.07mol) in ethanol, add triethylamine After reacting for 6 hours, the substituted product was separated by distillation under reduced pressure;
[0041]b. Dissolve the above substituted product in N,N'-dimethylformamide, the solute mass percentage concentration is 20%, after completely dissolving, cool to room temperature, add dianhydride (0.075mol), nitrogen protection, at 15°C Next, stir and react for 4 hours to obtain a polyamic acid solution;
[0042] c. add m-hydroxyaniline (0.00035mol), and react at room temperature for 3 hours to obtain a polyamic acid solution terminated with phenolic hydroxyl groups;
[0043] d. Add acetic anhydride and pyridine to carry out dehydration imidization reaction, react at 60°C for 8 hours, after the reaction is completed, precipitate, filter, wash, and dry in vacuum to obtain polyimide powder terminated with phenolic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com