Preparation method of all-solid polymer electrolyte and secondary lithium battery containing the electrolyte
An all-solid-state polymer, secondary lithium battery technology, applied in the field of lithium ion batteries, can solve problems such as easy leakage of liquids, and achieve the effects of solving fire and explosion, improving charging and discharging performance, and improving interface compatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
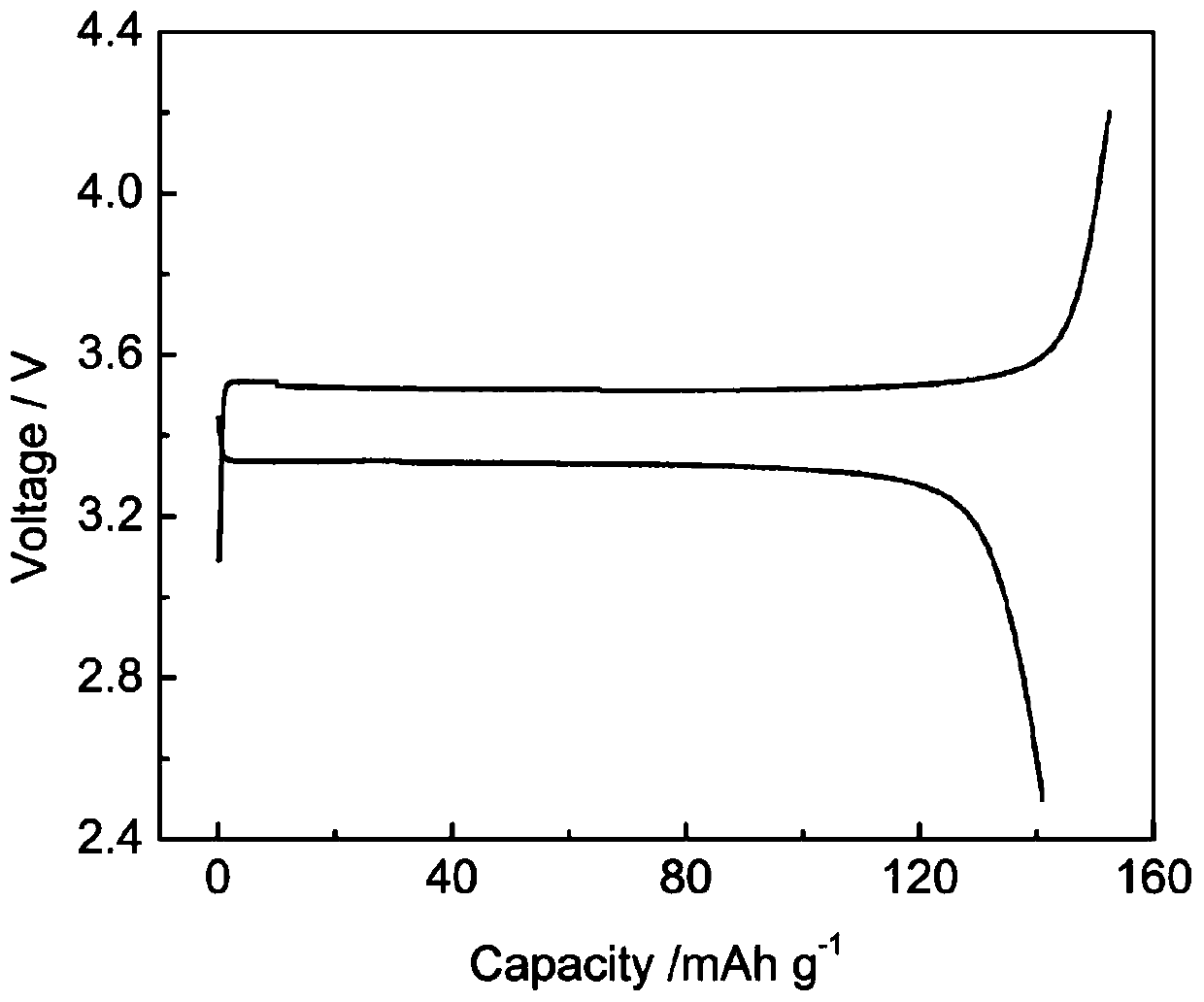
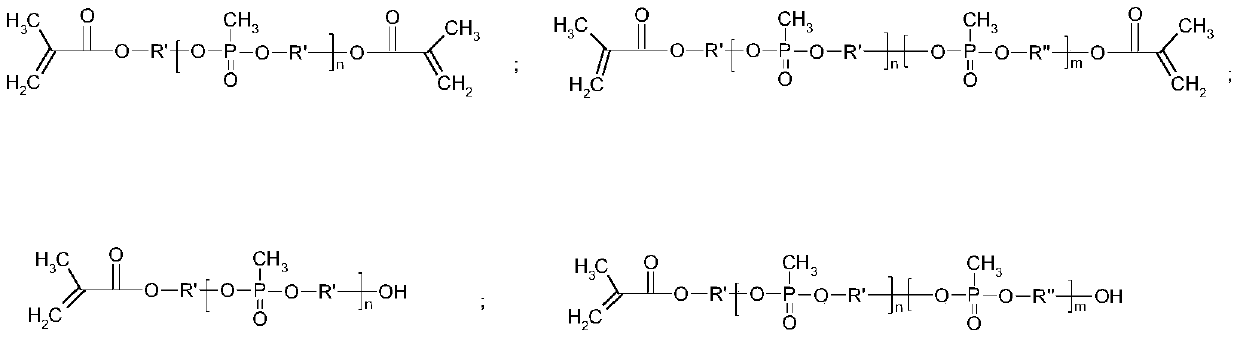
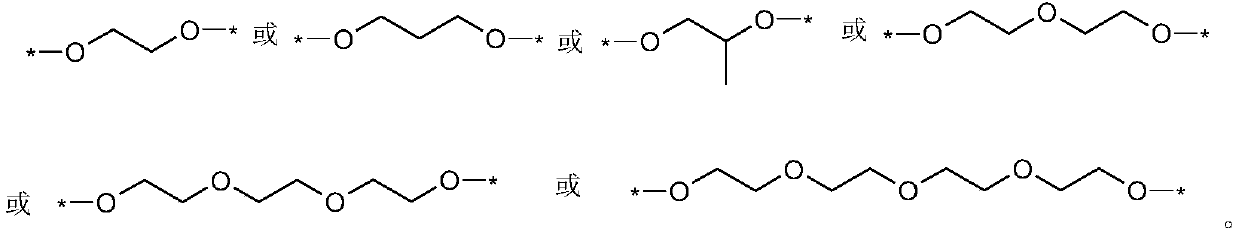
[0037] In a glove box filled with argon, dissolve LiTFSI in the double-ended dimethylacryloyloxypolymethylphosphonic acid (propylene glycol) ester monomer, add ammonium persulfate and magnetically stir for 4 hours to mix well; inject the well-mixed solution into Li / / SL (SL is a stainless steel pole piece), SL / / SL batteries were polymerized at 60°C for 4 hours, and then the ionic conductivity and electrochemical stability window of the all-solid polymer electrolyte were tested respectively.
[0038] Wherein, the mass ratio of double-ended dimethacryloyloxy polymethylphosphonic acid (propylene glycol) ester monomer, ammonium persulfate and LiTFSI is 100:0.4:35. The ratio of raw materials used to prepare solid polymer electrolytes is shown in Table 1, and the ion conductivity of the prepared polymer for lithium-ion batteries at room temperature is 1.2×10 -4 S / cm, the electrochemical window is 4.5V.
[0039] Table 1
[0040]
Embodiment 2
[0042] In an argon-filled glove box, LiTFSI was dissolved in double-ended dimethylacryloyloxypolymethylphosphonic acid (diethylene glycol) ester monomer, ammonium persulfate and lithium lanthanum zirconium oxide nanoparticles were added After magnetic stirring for 4 hours, mix well; inject the well-mixed solution into Li / / SL (SL is a stainless steel pole piece), put the SL / / SL battery at 60°C for 5 hours, and then test the ions of the all-solid polymer electrolyte Conductivity and electrochemical stability window.
[0043] Wherein, the mass ratio of double-ended dimethylacryloyloxypolymethylphosphonic acid (diethylene glycol) ester, ammonium persulfate, LiTFSI and lithium lanthanum zirconium oxide is 100:0.3:35:7. The ratio of the raw materials used to prepare the solid polymer electrolyte is shown in Table 2, and the ion conductivity of the prepared polymer for lithium-ion batteries at room temperature is 2.0×10 -4 S / cm, the electrochemical window is 4.5V.
[0044] Table 2 ...
Embodiment 3
[0047] In an argon-filled glove box, LiBOB was dissolved in a mixture of double-ended dimethylacryloyloxypolymethylphosphonic acid (propylene glycol) and single-ended dimethylacryloyloxypolymethylphosphonic acid (propylene glycol) In the body, add potassium persulfate and aluminum oxide nanoparticles and then magnetically stir for 4 hours to mix evenly; inject the evenly mixed solution into Li / / SL (SL is a stainless steel pole piece), and place the SL / / SL battery at 65°C After polymerization for 4 hours, the ionic conductivity and electrochemical stability window of the all-solid polymer electrolyte were tested respectively.
[0048] Among them, the mass ratio of double-ended epoxy-based polymethylphosphonic acid (propylene glycol) ester to single-ended epoxy-based polymethylphosphonic acid (propylene glycol) ester, potassium persulfate, LiBOB and aluminum oxide is 50:50: 0.2:32:7. The ratio of raw materials used to prepare solid polymer electrolytes is shown in Table 3, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com