Application of aescin and salt thereof in preparing medicine for treating cataracts
A escin and cataract technology, which is applied in drug delivery, drug combination, and pharmaceutical formula, etc., can solve the problems of complex pathogenesis, different treatment methods, and no cataract, etc., and achieve significant curative effect, good safety, and less irritation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 7
[0020] Example 1 Efficacy Test of Aescin and Its Salts in Treating Cataracts
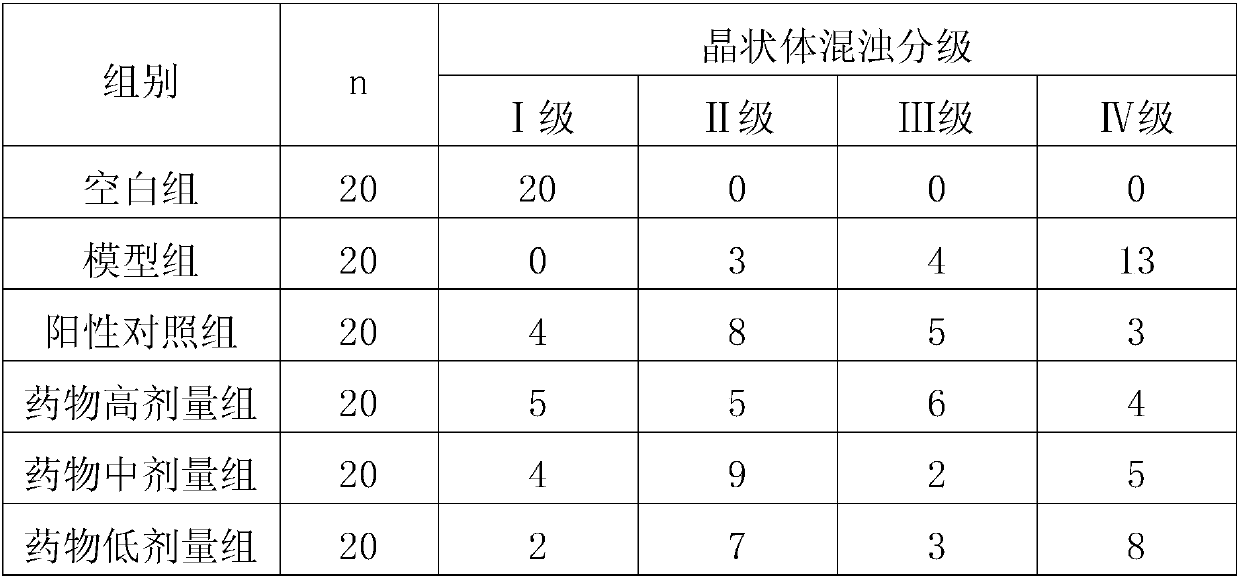
[0021] Test drugs: 50% D-galactose solution, Baineiting eye drops (0.05mg / ml), lysine aescin eye drops (0.5%, 0.8%, 1.2%).
[0022] Take 120 3-week-old pups, half male and half male, and randomly divide them into 6 groups, 20 rats in each group, namely: blank group, model group, positive control group, lysine aescin high, medium and low dose groups . Observing with a slit microscope before administration, those with transparent crystals are available for use; Modeling: Animals are injected intraperitoneally with 50% D-galactose solution 15g / kg every day, divided into two injections, with an interval of more than 4 hours, and continuously injected for 14 days. For animals in the blank group Normal saline (0.1mL / eye) was instilled in both eyes. The model group was given 1 drop of normal saline / eye, the positive control group was given 1 drop / eye of Baineiting eye drops, and the high, middle and low ...
Embodiment 2 7
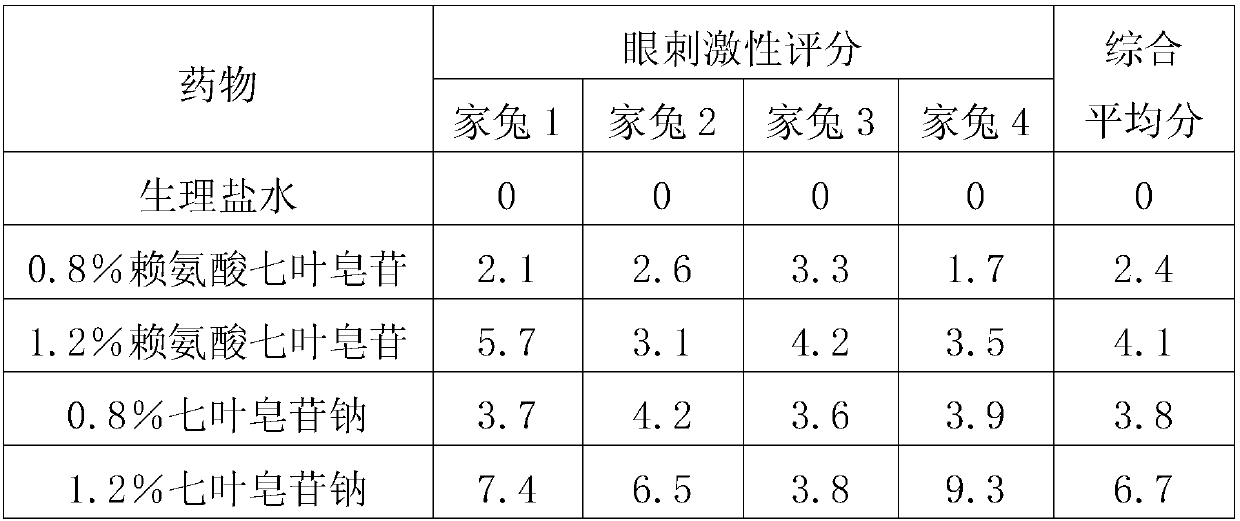
[0028] The eye irritation test of embodiment 2 aescin and its salt
[0029] Test drug:
[0030] 0.8% Lysine Aescin Eye Drops: Take 0.8g of lysine aescin, 1g of sodium chloride, 0.5g of borate, dissolve it in water for injection to 100g, filter it, and prepare it now after steam sterilization use.
[0031] 1.2% Lysine Aescin Eye Drops: Take 1.2g of lysine aescin, 1g of sodium chloride, 0.5g of borate, dissolve it in water for injection to 100g, filter it, and make it now after steam sterilization use.
[0032] 0.8% Sodium Aescinate Eye Drops: Take 0.8g of Sodium Aescinate, 1g of Sodium Chloride, and 0.5g of Borate, dissolve it in water for injection to 100g, filter it, and prepare it for immediate use after steam sterilization.
[0033] 1.2% Sodium Aescinate Eye Drops: Take 1.2g of Sodium Aescinate, 1g of Sodium Chloride, and 0.5g of Borate, dissolve it in water for injection to 100g, filter it, and prepare it for immediate use after steam sterilization.
[0034] 16 healthy...
Embodiment 3
[0038] Example 3 typical case
[0039]Wang Moumou, female, 73 years old, was first diagnosed in November 2015. Complaints of blurred vision in both eyes, photophobia, blurred vision. Check: binocular vision 0.13, conjunctiva without hyperemia, cornea transparent, pupil equal in size and round, good light reflection, iris and ciliary body without hyperemia, lens is off-white with different turbidity, and vitreous body is not turbid. There was no abnormality in fundus peeping, and it was diagnosed as the terminal stage of senile cataract in both eyes. After 21 days of treatment with lysine aescin eye drops, the binocular vision improved to 0.5, and 30 days was a course of treatment, and the binocular vision improved to 0.7. Slit-lamp examination after pupil dilation showed that the lens opacity disappeared, and the vision was stable after 6 months of follow-up.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mild irritant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com