Content determination method for orifice-freeing rhinitis tablet
A technology of Tongqiao Biyan Tablet and its determination method, which is applied in the field of content determination of Chinese patent medicines, can solve the problems that the six active ingredients of Tongqiao Biyan Tablet cannot be detected at the same time, the preparation method is complicated, and takes a long time, so as to shorten the time of content determination and improve the The effect of detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
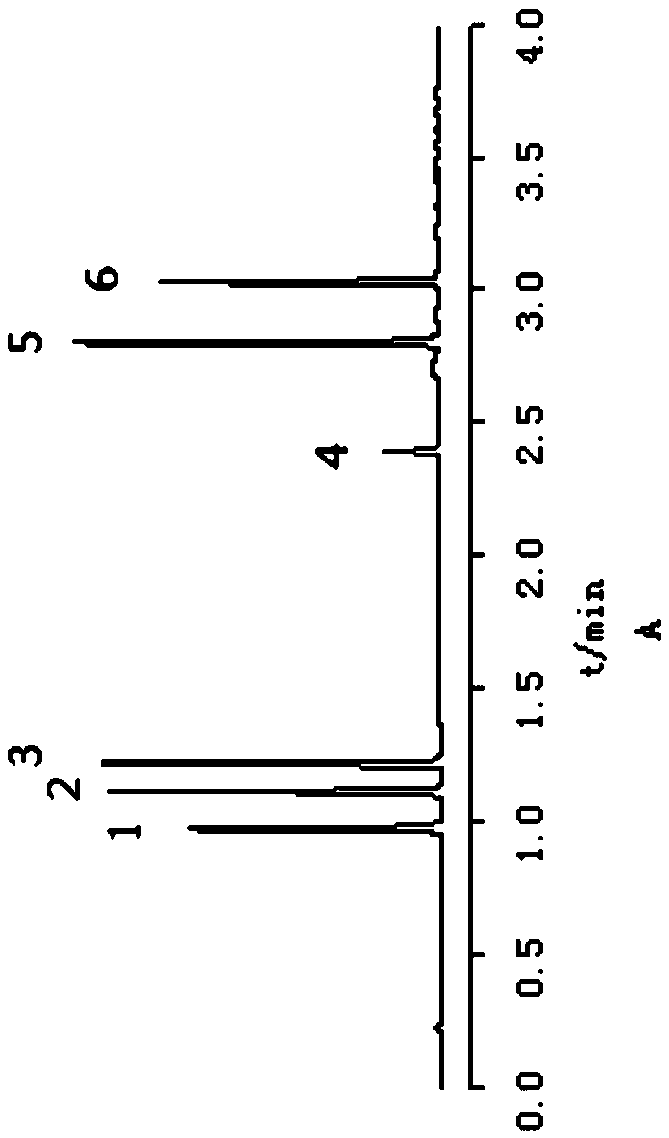
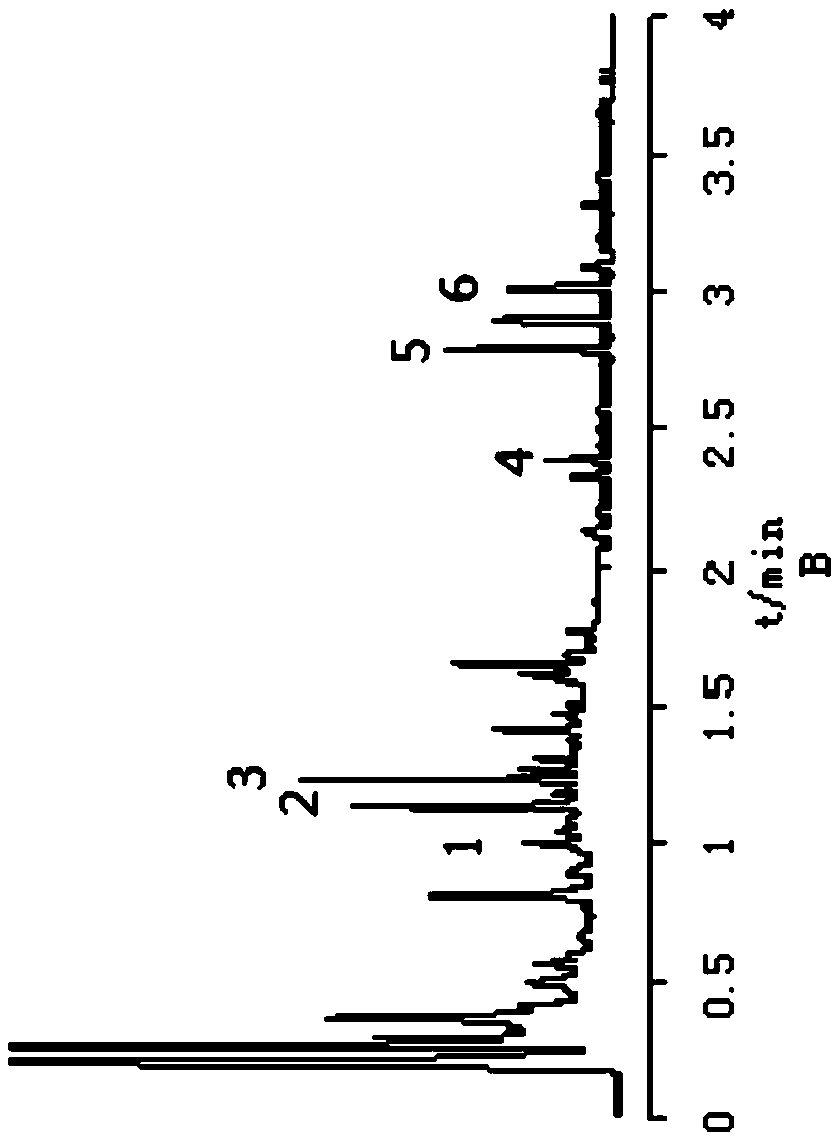
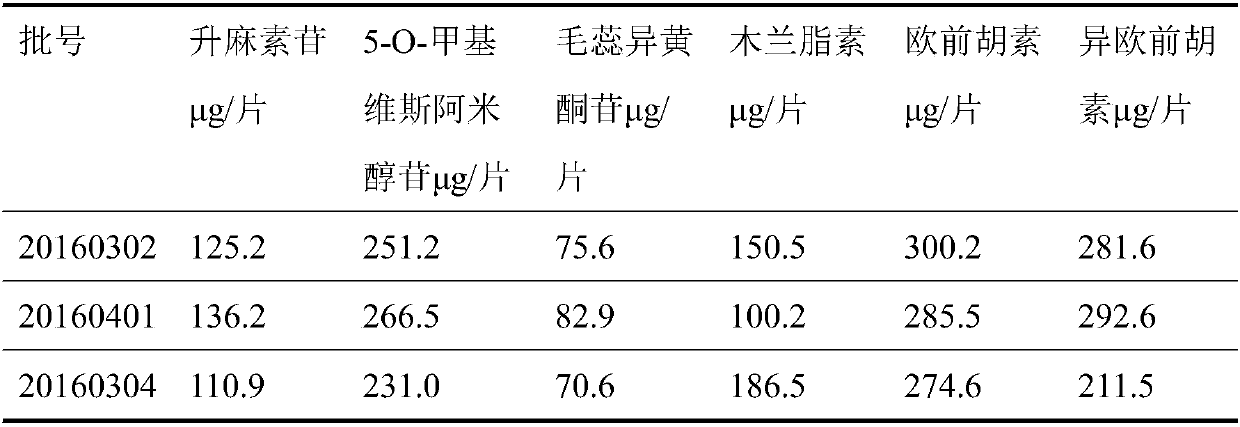
[0046] Example 1 The active ingredients acteoside, 5-O-methylvisamigoside, acteosin, magnolanin, imperatorin, and isoimperatorin in Tongqiao Biyan Tablets were detected according to the following method. content:
[0047](1) Preparation of the test solution: take 1.0 g of Tongqiao Biyan Tablet powder, accurately weigh it, put it in a 100 mL conical flask with a stopper, accurately add 50 mL of methanol with a volume fraction of 80%, weigh, extract by 100W, 25kHz ultrasound for 45min, and put Cool, weigh again, make up for the weight loss with 80% methanol by volume fraction, filter with a 0.22 μm microporous membrane, and take the subsequent filtrate as the test solution;
[0048] (2) Preparation of reference solution: take acteoside reference substance, 5-O-methylvisamidol glycoside reference substance, calycosin glucoside reference substance, imperatorin reference substance, isoimperatorin The plain reference substance and the magnolanin reference substance were dissolved i...
Embodiment 2
[0053] Example 2 The active ingredients in Tongqiao Biyan Tablets were detected according to the following method: acteoside, 5-O-methyl visamidol glycoside, acteoisoflavone glycoside, magnolanin, imperatorin, isoimperatorin content:
[0054] (1) Preparation of the test solution: take 3.0 g of Tongqiao Biyan Tablet powder, accurately weigh it, put it in a 200 mL volumetric flask, add 30 mL of methanol with a volume fraction of 60% accurately, weigh it, extract it with 100 W, 25 kHz ultrasonic wave for 30 min, let it cool, Weigh again, make up for the weight loss with 60% methanol by volume fraction, filter with a 0.22 μm microporous membrane, and take the subsequent filtrate as the test solution;
[0055] (2) Preparation of reference solution: take acteoside reference substance, 5-O-methylvisamidol glycoside reference substance, calycosin glucoside reference substance, imperatorin reference substance, isoimperatorin The plain reference substance and the magnolanin reference s...
Embodiment 3
[0060] Example 3 The active ingredients acteoside, 5-O-methyl visamidol glycoside, acteoisoflavone glycoside, magnolanin, imperatorin, and isoimperatorin in Tongqiao Biyan Tablets were detected according to the following method. content:
[0061] (1) Preparation of the test solution: Take 2.0g of Tongqiao Biyan Tablet powder, accurately weigh it, put it in a 150mL conical flask with a stopper, add 60mL of methanol with a volume fraction of 70% precisely, weigh it, extract it with 100W, 25kHz ultrasound for 60min, put Cool, weigh again, make up for the weight loss with 70% methanol by volume fraction, filter with a 0.22 μm microporous membrane, and take the subsequent filtrate as the test solution;
[0062] (2) Preparation of reference solution: take acteoside reference substance, 5-O-methylvisamidol glycoside reference substance, calycosin glucoside reference substance, imperatorin reference substance, isoimperatorin The plain reference substance and the magnolanin reference ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com