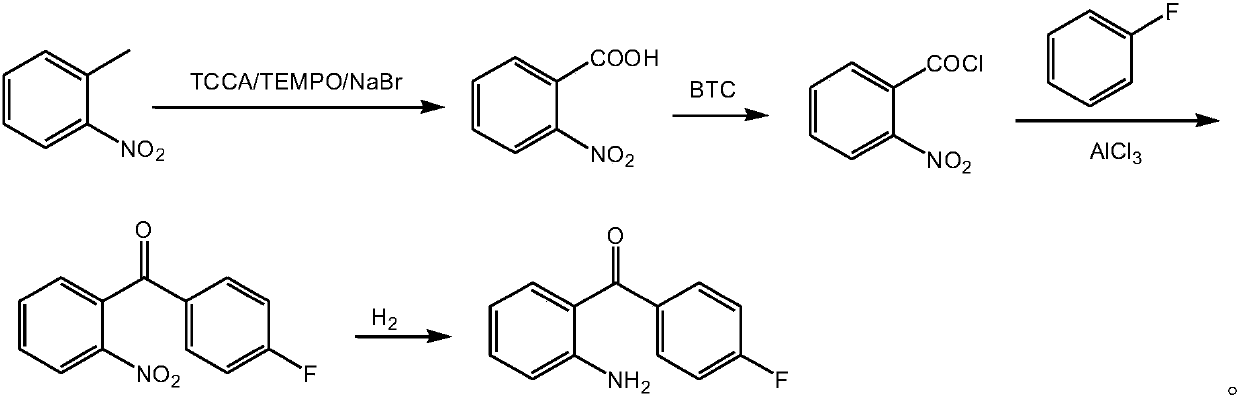
Method for preparing 2-amino-4'-fluorobenzophenone
A technology of benzophenone and o-nitrobenzoic acid, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of low total yield, many reagents, complicated operation, etc., and meet the reaction conditions Moderate and controllable, convenient and controllable operation, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A method for preparing 2-amino-4'-fluoro-benzophenone, comprising the following steps: performing an oxidation reaction with o-nitrotoluene, trichloroisocyanuric acid, tetramethylpiperidine nitrogen oxide, and sodium bromide to obtain O-nitrobenzoic acid; followed by acyl chloride reaction with trichloromethyl carbonate to obtain o-nitrobenzoyl chloride; then Friedel-Crafts reaction with fluorobenzene and aluminum trichloride to obtain 2-nitro-4'-fluoro- Benzophenone; finally get 2-amino-4'-fluoro-benzophenone by hydrogen reduction.
Embodiment 2
[0049] A method for preparing 2-amino-4'-fluoro-benzophenone, comprising the steps of:
[0050] Take o-nitrotoluene, tetramethylpiperidine nitrogen oxide, sodium bromide, and the reaction solvent for the oxidation reaction, raise the temperature to 0°C, add trichloroisocyanuric acid, keep it warm for 24 hours, quench the reaction with methanol, and filter to get the filtrate. Extract the organic layer with dichloromethane, wash, dry, concentrate under reduced pressure, recrystallize with a mixed solvent of ethyl acetate and sherwood oil to obtain o-nitrobenzoic acid, wherein the reaction solvent is a mixed solvent of dichloromethane and water, Among them, the volume ratio of dichloromethane and water is 10:1, the molar ratio of trichloroisocyanuric acid, tetramethylpiperidine nitrogen oxide, and sodium bromide is 1:0.001:0.2, o-nitrotoluene and trichloro The molar ratio of isocyanuric acid is 1:0.8;
[0051] O-nitrobenzoic acid and trichloromethyl carbonate are carried out un...
Embodiment 3
[0055] A method for preparing 2-amino-4'-fluoro-benzophenone, comprising the steps of:
[0056] Take o-nitrotoluene, tetramethylpiperidine nitrogen oxide, sodium bromide, and the reaction solvent for the oxidation reaction, raise the temperature to 30°C, add trichloroisocyanuric acid, keep it warm for 10 hours, quench the reaction with methanol, and filter to obtain the filtrate. Extract the organic layer with dichloromethane, wash, dry, concentrate under reduced pressure, recrystallize with a mixed solvent of ethyl acetate and sherwood oil to obtain o-nitrobenzoic acid, wherein the reaction solvent is a mixed solvent of dichloromethane and water, Among them, the volume ratio of dichloromethane and water is 10:1, the molar ratio of trichloroisocyanuric acid, tetramethylpiperidine nitrogen oxide, and sodium bromide is 1:0.1:0.05, o-nitrotoluene and trichloro The molar ratio of isocyanuric acid is 1:2;
[0057] Mix o-nitrobenzoic acid, trichloromethyl carbonate, triethylenediam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com