Layer-by-layer self-assembly body having both tumor suppression and bone defect repair functions and preparation method thereof
A layer-by-layer self-assembly and bone defect technology, which is applied in the field of tissue engineering bone repair and biomedical materials, can solve problems such as uneven distribution, denaturation, protein aggregation, etc., and achieve a simple preparation process, avoid environmental pollution, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
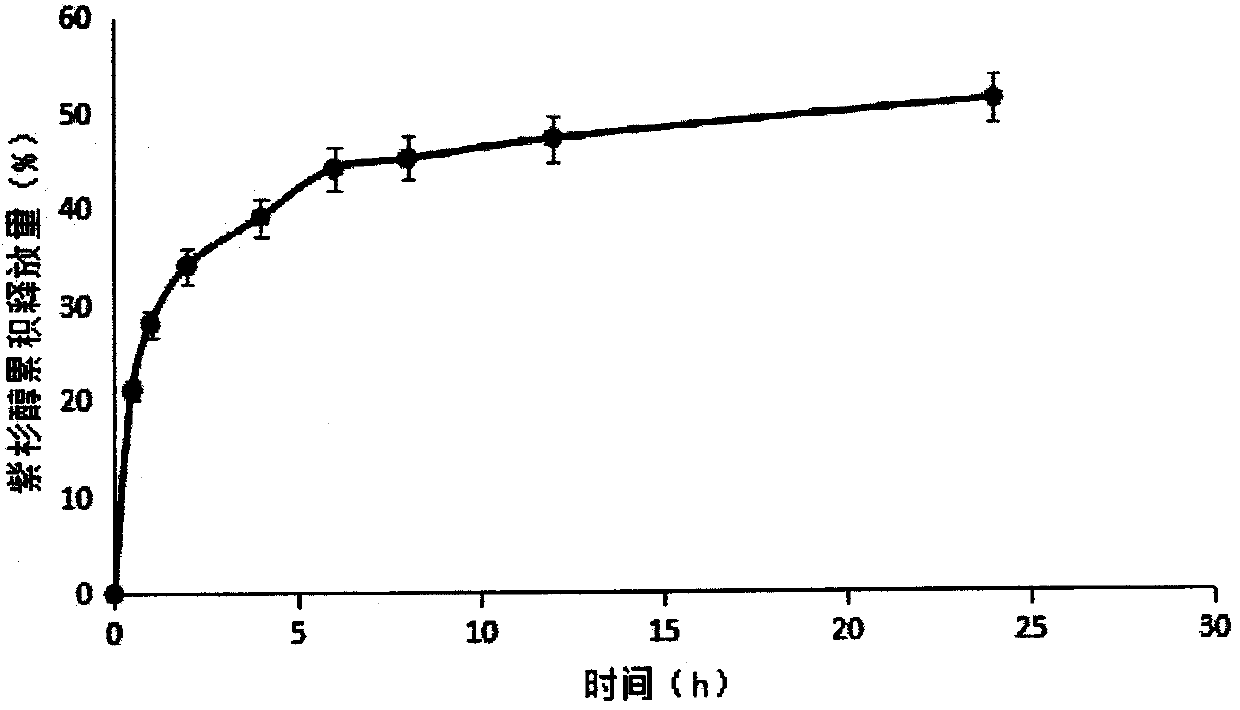
Image
Examples
Embodiment 1
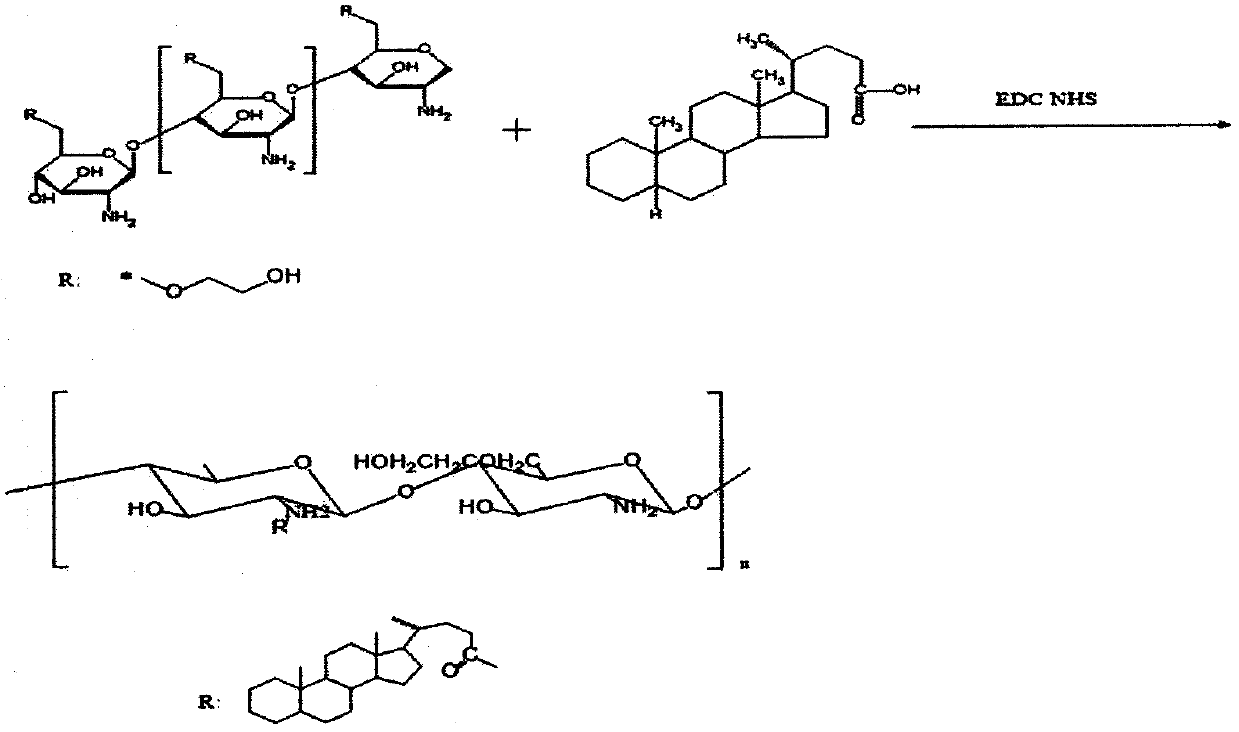
[0041] (1) Preparation of 5β-cholane acidified glycol chitosan (HGC): 0.15g glycol chitosan (GC) and 0.0037g ethyl-dimethylamine-propyl carbodiimide ( EDC) was dissolved in 18ml distilled water, then 0.0045g 5β-cholanic acid (5β-CA) and 0.0022g N-hydroxysuccinimide (NHS) were dissolved in 18ml methanol, and the 5β-CA solution was slowly added dropwise to In the GC solution under stirring state, the solution was stirred at room temperature for 24 hours until the solution became transparent, and dialyzed with a dialysis bag (14KDa) for 3 days, followed by dialysis with 80% methanol, 50% methanol, and pure water for one day, and changed every 3 hours. Once the dialysate, the dialyzed solution is filtered with a 0.8 μm filter membrane, freeze-dried to obtain the white flocculent product (HGC) of 5β-cholane acidified glycol chitosan, and its preparation process is shown in figure 1 .
[0042] (2) Preparation of paclitaxel nanoparticles loaded on 5β-cholane acidified ethylene glyco...
Embodiment 2
[0045] (1) Preparation of 5β-cholane acidified glycol chitosan (HGC): 0.15g glycol chitosan (GC) and 0.0048g ethyl-dimethylamine-propyl carbodiimide ( EDC) was dissolved in 18ml of distilled water, then 0.0060g of 5β-cholanic acid (5β-CA) and 0.0029g of N-hydroxysuccinimide (NHS) were dissolved in 18ml of methanol, and the 5β-CA solution was slowly added dropwise to In the GC solution under stirring state, the solution was stirred at room temperature for 24 hours until the solution became transparent, and dialyzed with a dialysis bag (14KDa) for 3 days, followed by dialysis with 80% methanol, 50% methanol, and pure water for one day, and changed every 3 hours. Dialysate once, the dialyzed solution was filtered with a 0.8 μm filter membrane, and freeze-dried to obtain a white flocculent product (HGC) of 5β-cholaneated glycol chitosan.
[0046] (2) Preparation of paclitaxel nanoparticles loaded on 5β-cholane acidified ethylene glycol chitosan (HGC-PTX): Slowly add paclitaxel PTX...
Embodiment 3
[0049] (1) Preparation of 5β-cholane acidified glycol chitosan (HGC): 0.15g glycol chitosan (GC) and 0.0074g ethyl-dimethylamine-propyl carbodiimide ( EDC) was dissolved in 18ml of distilled water, then 0.0090g of 5β-cholanic acid (5β-CA) and 0.0045g of N-hydroxysuccinimide (NHS) were dissolved in 18ml of methanol, and the 5β-CA solution was slowly added dropwise to In the GC solution under stirring state, the solution was stirred at room temperature for 24 hours until the solution became transparent, and dialyzed with a dialysis bag (14KDa) for 3 days, followed by dialysis with 80% methanol, 50% methanol, and pure water for one day, and changed every 3 hours. Dialysate once, the dialyzed solution was filtered with a 0.8 μm filter membrane, and freeze-dried to obtain a white flocculent product (HGC) of 5β-cholaneated glycol chitosan.
[0050] (2) Preparation of paclitaxel nanoparticles loaded on 5β-cholane acidified ethylene glycol chitosan (HGC-PTX): Slowly add paclitaxel PTX...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com