Method for synthesizing NADPH through immobilized enzyme catalysis
A technology for immobilizing enzymes and kinases, applied in the directions of immobilizing enzymes, biochemical equipment and methods, enzymes, etc., can solve the problems of cumbersome synthesis steps, long production cycle and high production cost, achieve high recovery rate of enzyme activity and simplify production. Process, high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
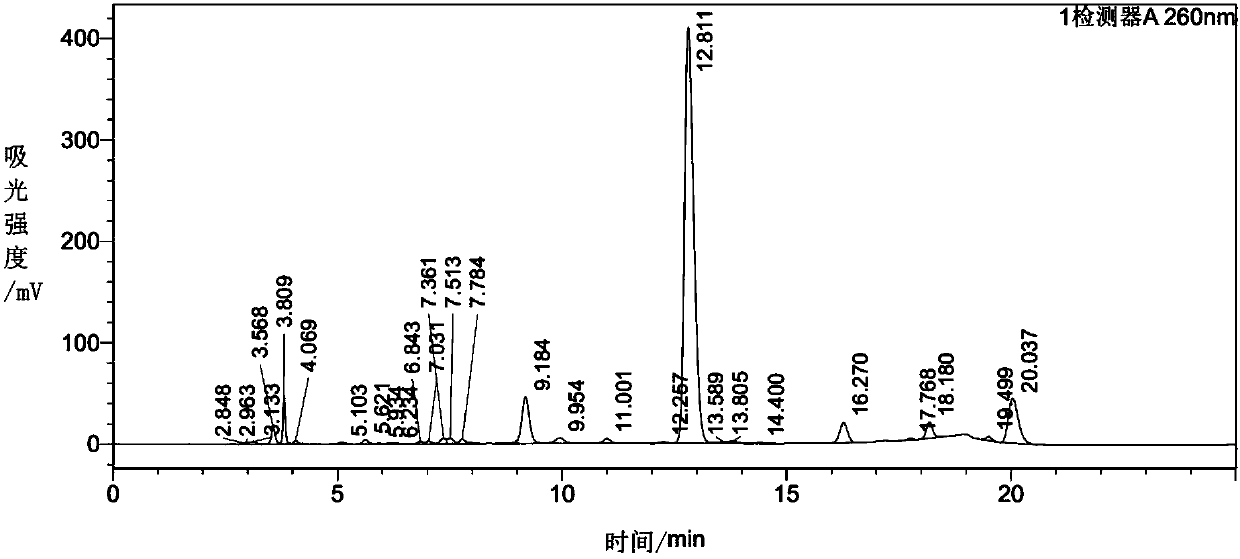
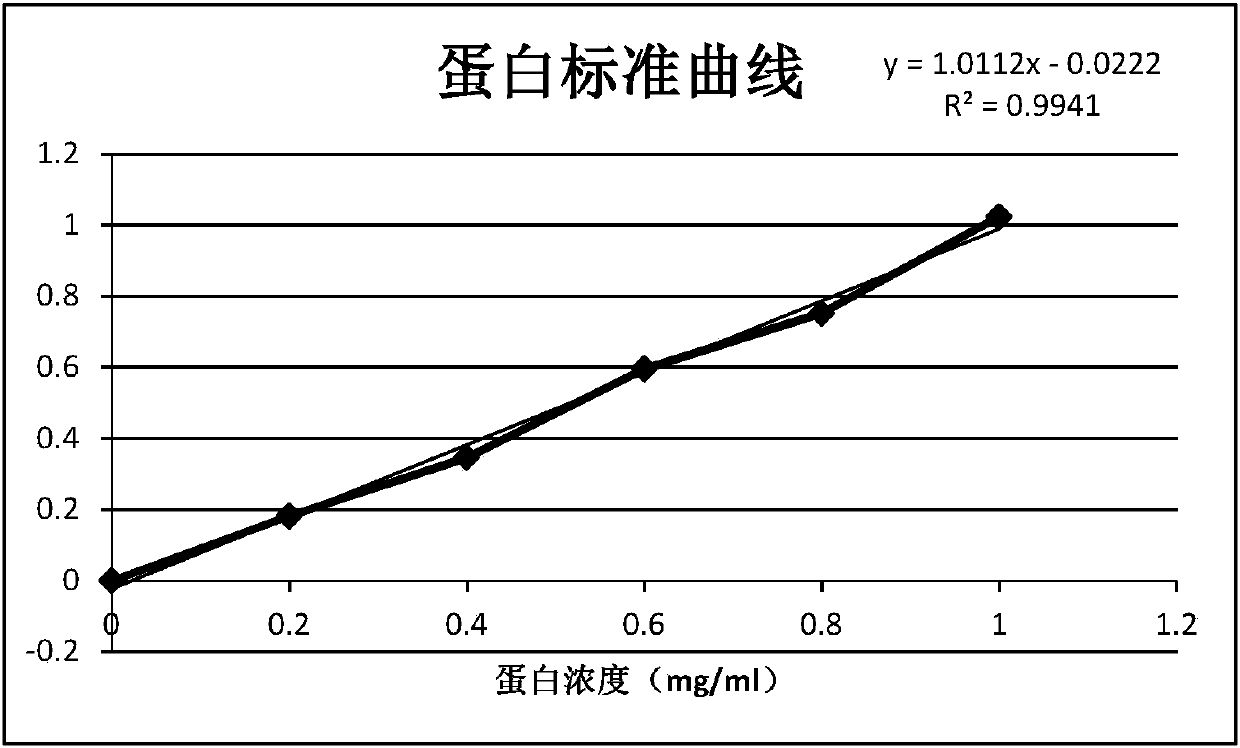
Image
Examples
Embodiment 1
[0047] The invention provides a preparation method of immobilized NAD kinase, specifically comprising the following steps:
[0048] 1. Preparation of NAD kinase-producing bacteria
[0049] Artificially synthesized NAD kinase expression gene derived from Mycobacterium tuberculosis (Mycobacterium tuberculosis), the gene sequence is shown in SEQ ID NO.1, which is connected to the pET24a vector (Novagen Company, 69749-3) through NdeI and HindIII sites, and sequenced After being correct, it was loaded into Ecoli BL21 strain, and the high-expression strain was screened as the NAD kinase producer.
[0050] 2. Preparation of NAD kinase
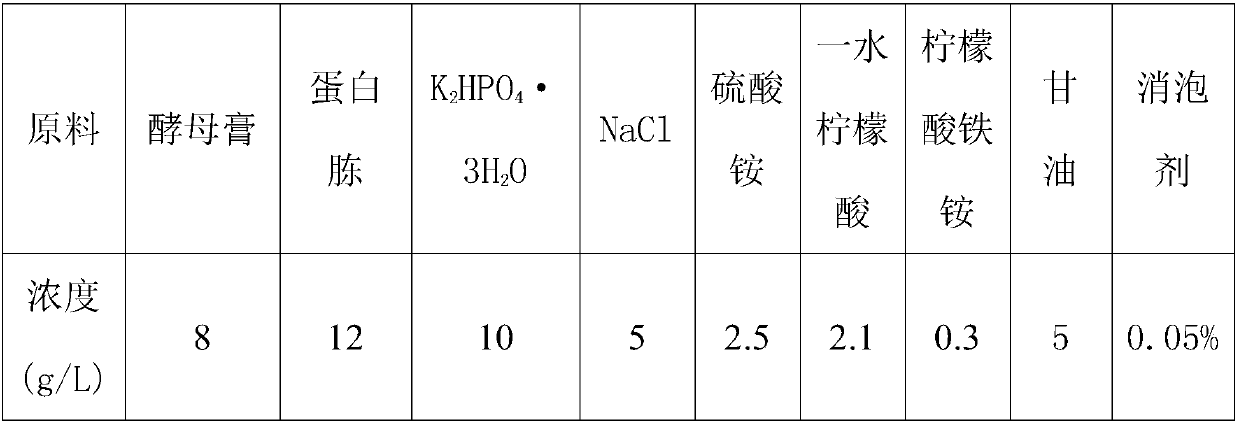
[0051] (1) Melt the strain tube with NAD kinase producing bacteria at room temperature, then use an inoculation loop to dip a ring and draw a line on the seed medium (kanamycin sulfate 5mg / L) plate containing kanamycin sulfate, The composition of the seed medium is shown in Table 1, and it was activated overnight at 37°C.
[0052] Table 1 Seed Medi...
Embodiment 2
[0071] The present invention provides a preparation method of immobilized NAD kinase, which differs from the preparation method shown in Example 1 only in that:
[0072] 1. Add glutaraldehyde with a mass concentration of 1% to the enzyme carrier LX-1000HFA to activate for 1.5 hours, and filter to obtain the activated carrier.
[0073] 2. Add the activated LX-1000HFA to the enzyme solution prepared in Implementation 1 at a mass ratio of protein: carrier = 1:5 (or wet bacteria: carrier = 1:2), and then adjust the pH to 5.0. Stir slowly at 100 rpm at a temperature of 30°C, and fix for 25 hours.
Embodiment 3
[0075] The invention provides a kind of preparation method of immobilized glucose dehydrogenase, specifically comprises the following steps:
[0076] 1. Preparation of glucose dehydrogenase-producing bacteria
[0077]Artificially synthesize the glucose dehydrogenase expression gene derived from Bacillus subtilis (Bacillus subtilis), the gene sequence is as shown in SEQ ID NO.2, and it is connected to the pET24a vector (Novagen Company, 69749-3) through the NdeI and HindIII sites, After the sequence was correct, it was loaded into the Ecoli BL21 strain, and the high-expression strain was screened as the glucose dehydrogenase-producing strain.
[0078] 2. Preparation of glucose dehydrogenase
[0079] According to the preparation method provided in Example 1, glucose dehydrogenase enzyme solution was prepared by using glucose dehydrogenase-producing bacteria.
[0080] 3. Preparation of immobilized glucose dehydrogenase
[0081] According to the preparation method provided in E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com