Allyl imine bridged ferrocene-rhodamine B multichannel response receptor molecule and synthesizing method and application thereof
A technology for bridging ferrocene and acceptor molecules with allyl imine, which is applied in the directions of chemical reaction of materials, material analysis by observing the influence on chemical indicators, chemical instruments and methods, etc. Solve the problems of limited application scope and single detection method, and achieve the effect of improving detection sensitivity, fast response, and increasing binding sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] In a preferred embodiment of the present invention, a method for synthesizing the above-mentioned allylimine bridged ferrocene-rhodamine B multichannel response receptor molecule is also provided, which method includes rhodamine B spironolactone hydrazide and acetylvinyl A step in which ferrocene is condensed to form the acceptor molecule.
[0030] Among them, rhodamine B spironolide hydrazide and acetylvinylferrocene are existing substances that can be prepared by those skilled in the art through conventional methods, and no special limitation is required here. For example, rhodamine B spironolide hydrazide can be prepared by the method in the patent (CN 106323893A); , C.; Wu, S.; Wang, D.H.Inorg.Chem.2008, 47, 7190-7201.) prepared by the method provided.
[0031] In a preferred embodiment of the present invention, the preparation of the Rhodamine B spironolide hydrazide: Weigh a certain amount of Rhodamine B, dissolve it with ethanol, add excess hydrazine hydrate (80...
Embodiment 1
[0060] Synthesis of receptor molecules
[0061] In a 25mL two-necked flask equipped with a stirring magnet and a spherical condenser, add rhodamine B spironolide hydrazide (0.228g, 0.5mmol), acetylvinyl ferrocene (0.127g, 0.5mmol), ethanol ( 4mL) and glacial acetic acid (0.01mL), the mixture was stirred and heated to react (80°C), tracked by thin layer chromatography, after the reaction was complete, extracted with ethyl acetate, and the organic phase was washed successively with saturated sodium bicarbonate and saturated brine, Dry over anhydrous magnesium sulfate, separate by column chromatography (ethyl acetate / petroleum ether=1:3, R f =0.5), to obtain 0.297g of orange powdery solid (acceptor molecule of the present invention), yield rate is 86%, m.p.225-226 ℃).IR (cm –1 ):v max 1687(C=O), 1611(C=N). 1 H NMR (300MHz, CDCl 3 ,TMS):δ7.96-7.93(m,1H),7.50-7.47(m,2H),7.17-7.15(m,1H),6.81(d,1H,J=16.3Hz),6.59(d,2H ,J=8.7Hz),6.48(d,1H,J=16.4Hz),6.41(s,2H),6.32(d,2H,J=8.8Hz),4...
Embodiment 2
[0063] Recognition effect of acceptor molecules on various tested ions in the ultraviolet-visible spectrum detection embodiment 1
[0064] Prepare 5×10 in a 10mL volumetric flask -4 Receptor molecule standard solution of M, prepare 1×10 in a 10mL volumetric flask -3 Different ion aqueous solutions of M to be tested; respectively pipette 100 μL 5×10 in the colorimetric tube -4 M receptor molecule standard solution and 50 μL of the above-mentioned ion aqueous solution to be tested, with H 2 After the volume of O / THF (9:1, v:v) was adjusted to 5 mL, the UV-Vis absorption intensity was measured respectively.
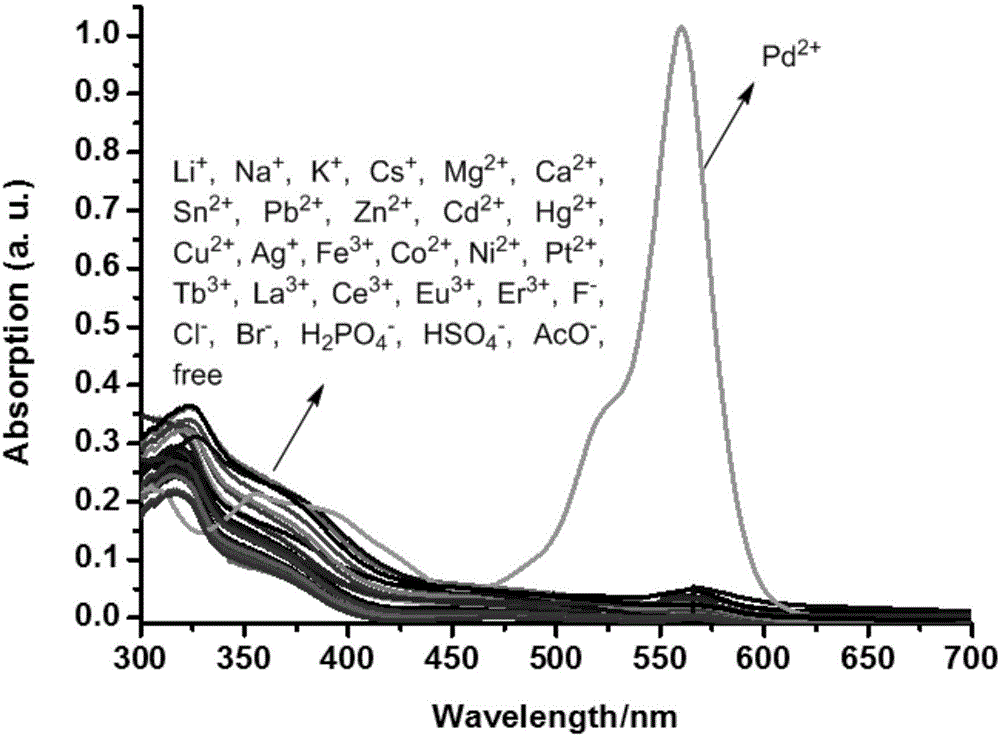
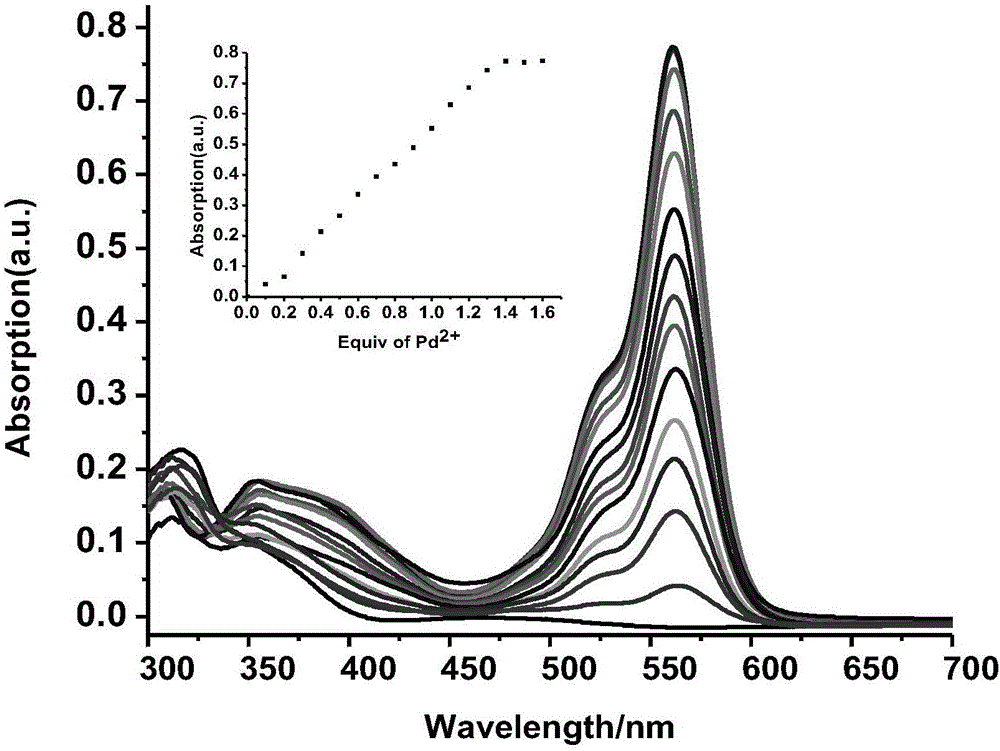
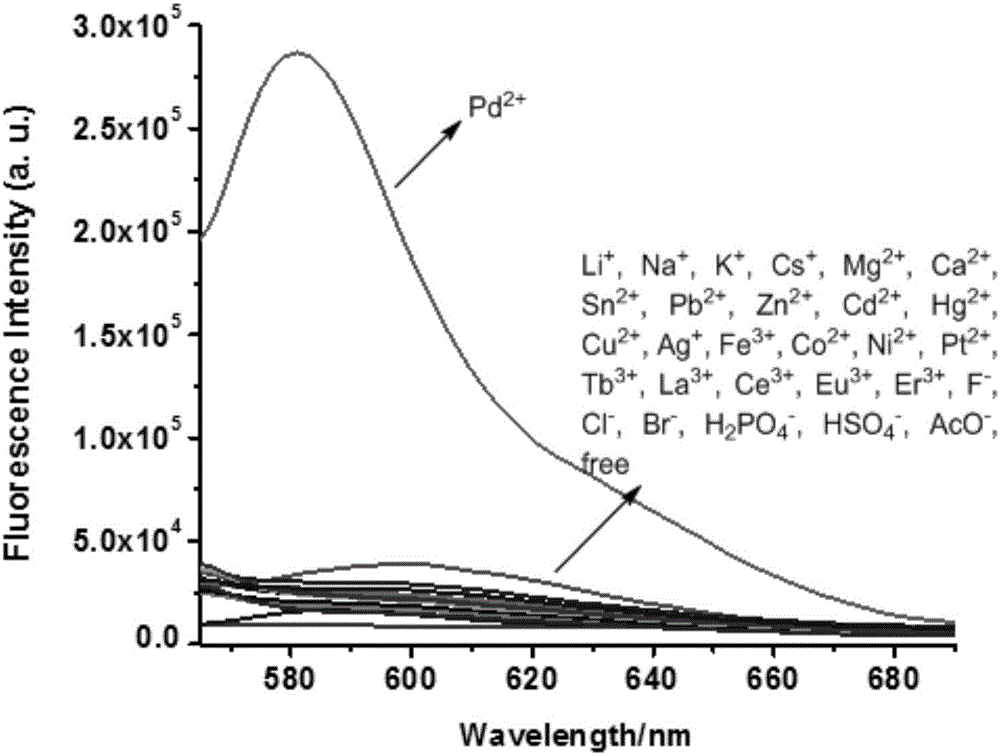
[0065] The result is as figure 1 , indicating that only the addition of Pd 2+ ions, the UV-visible absorption of the acceptor molecule changes significantly, and a new strong absorption peak appears at 562nm, indicating that the acceptor molecule can be highly selective with Pd 2+ ion binding.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com