Chemically constructed multifunctional non-viral gene conveying carrier polymer based on thiolactone
A technology of polymers and compounds, applied in gene therapy, genetic material components, and other methods of inserting foreign genetic materials, etc., to achieve the effects of wide application prospects, low cytotoxicity, and high transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The synthetic method of embodiment 1 compound 1, 2 and 3
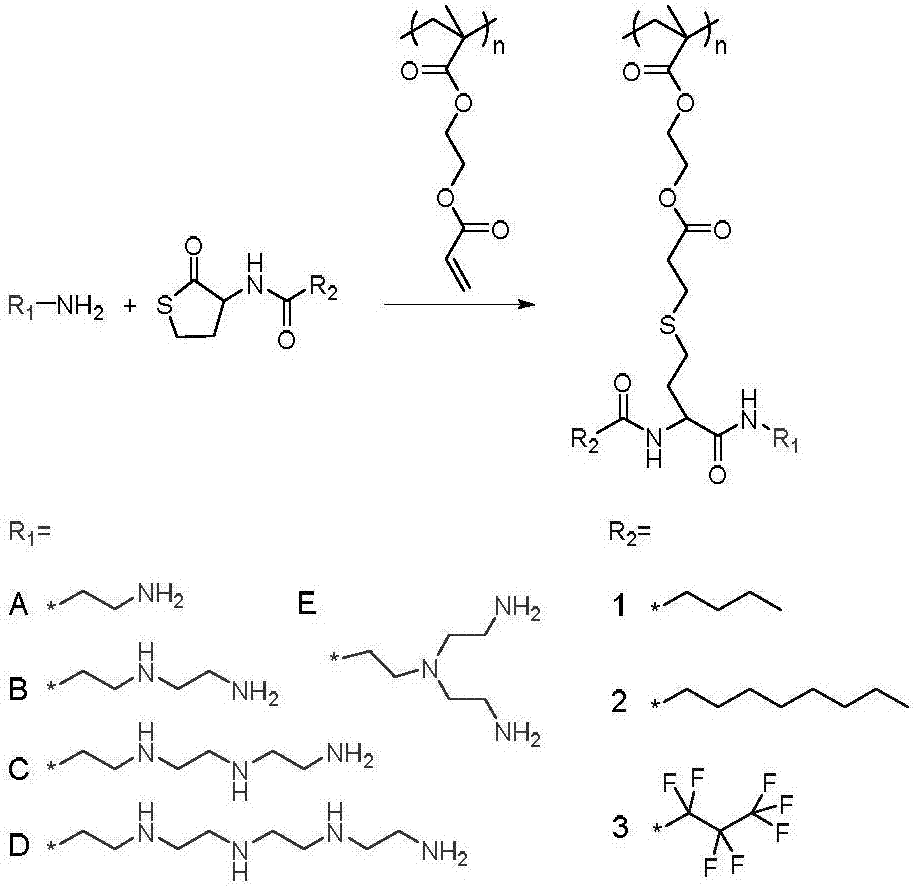
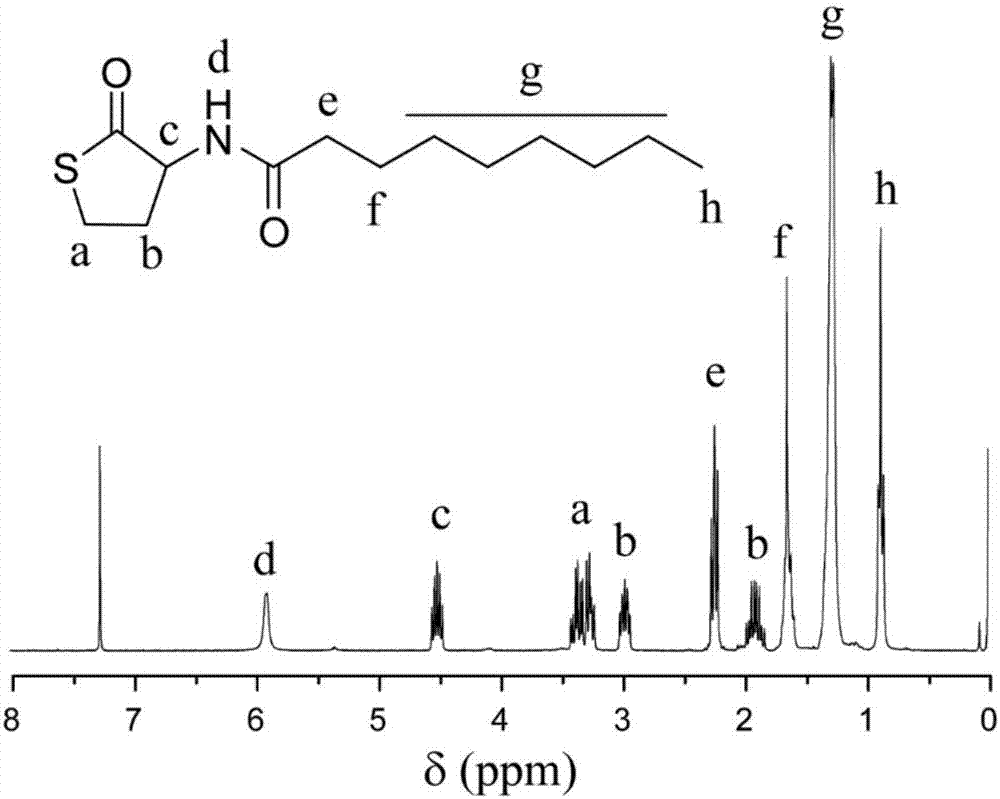
[0048] Through the amidation reaction between D, L-homocysteine thiolactone hydrochloride and n-valeric acid, n-nonanoic acid and perfluorobutyric acid, thiolactone compounds with different types of hydrophobic moieties were synthesized (respectively Recorded as: 1,2,3). Taking the synthesis of compound 1 as an example, at first, 100 mL of dichloromethane (DCM) containing D, L-homocysteine sulfate lactone hydrochloride (6.12g, 40mmol), triethylamine (7mL, 50mmol) at room temperature Under stirring, then add n-nonanoic acid (7.0g, 45mmol), dicyclohexylcarbodiimide (DCC) (9.2g, 45mmol) and dimethylaminopyridine (DMAP) (550mg, 4.5mmol), continue the reaction mixture Stir at room temperature for 24 hours. Next, the reactant was suction-filtered under reduced pressure to remove the insoluble residue, washed with ethyl acetate and filtered several times. After the solvent was evaporated under reduced pressure, ...
Embodiment 2
[0049] The synthetic method of embodiment 2 polyacryloxyethyl methacrylate (PAOEMA)
[0050] Such as Figure 5 As shown, PAOEMA is synthesized by post-modification of poly(2-hydroxyethyl methacrylate) (PHEMA) with acryloyl chloride. First, we synthesized PHEMA by reversible addition-fragmentation chain transfer (RAFT) polymerization. Specifically, the monomer 2-hydroxyethyl methacrylate (HEMA) (5.06 g, 40 mmol), the chain transfer agent (4-cyano-4-[(phenethylsulfanylthiocarbonyl)sulfur Alkyl]valeric acid) (155 mg, 0.45 mmol) and initiator azobisisobutyronitrile (AIBN) (6.5 mg, 0.04 mmol) were dissolved in absolute ethanol (5 mL) and transferred to a locked tube. After the reaction mixture was subjected to three freeze-thaw cycles to remove air, the sealed tube was sealed under vacuum, and the sealed tube was immersed in an oil bath preheated to 75° C., and the polymerization reaction was carried out under magnetic stirring. After 12 hours, the reaction mixture was precipita...
Embodiment 3
[0051] The multifunctional modification method of embodiment 3 series polymers
[0052] The multifunctional polymer mainly uses amines containing different numbers of ethyleneimine repeating units to carry out ring-opening reaction on thiolactones containing hydrophobic functional groups, and the sulfhydryl groups generated in situ after ammonolysis undergo Michael addition reaction with PAOEMA. Taking the synthesis of P2B as an example, first, a DMF solution containing 2 equivalents (relative to the double bond in PAOEMA, the same below) of compound 2 (431mg, 1.68mmol) was added dropwise to 20 equivalents of diethylenetriamine (1.73g, 16.8 mmol) in DMF solution. After stirring the reaction at room temperature for 10 minutes, the 2 A DMF solution containing PAOEMA (150 mg, 0.01 mmol) was slowly added to the system under atmosphere. After continuing the reaction for 15 minutes, the reaction solution was precipitated and centrifuged three times in ether to finally obtain the l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com