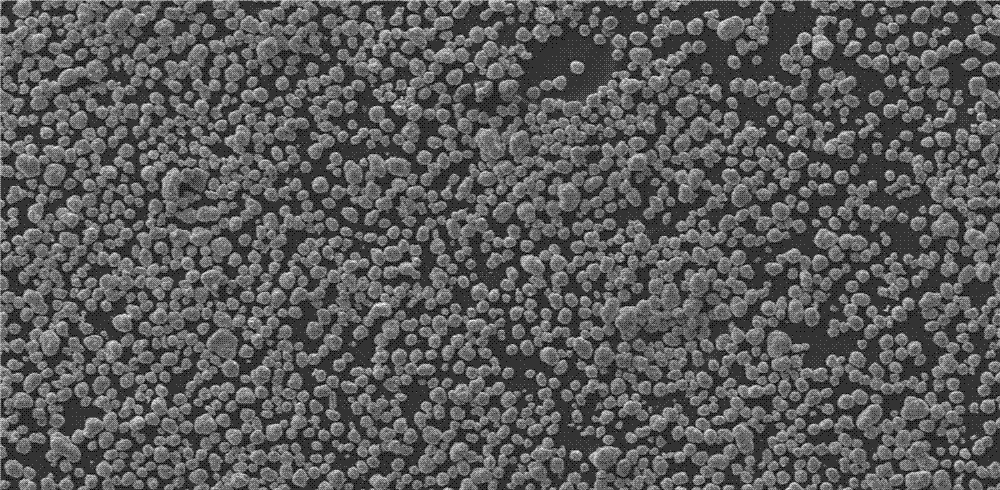
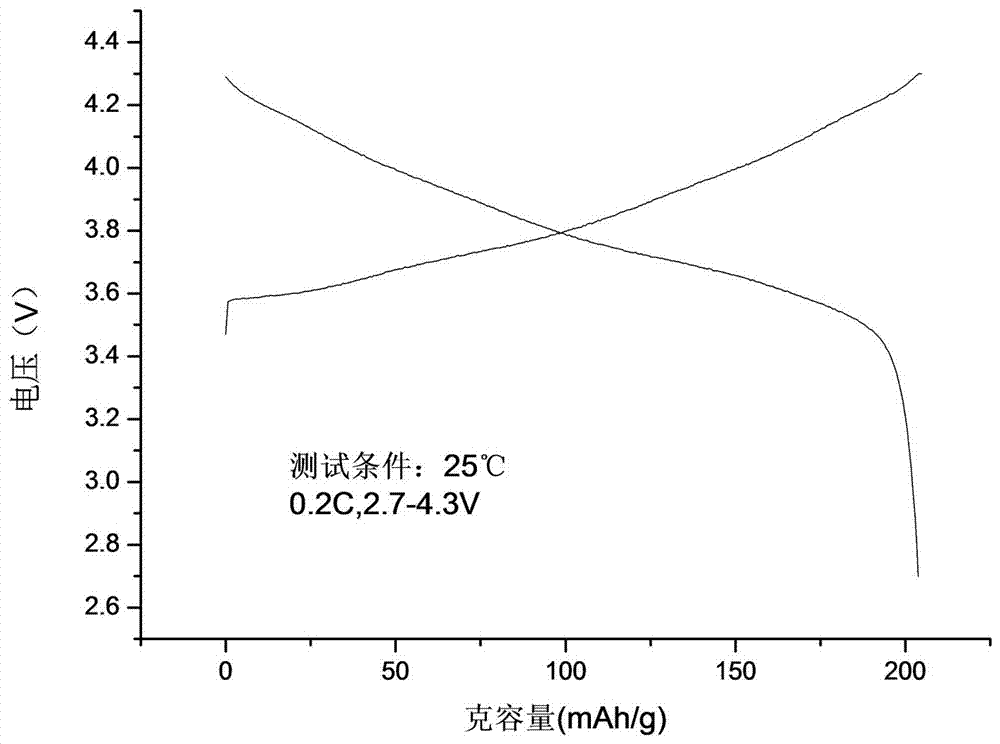
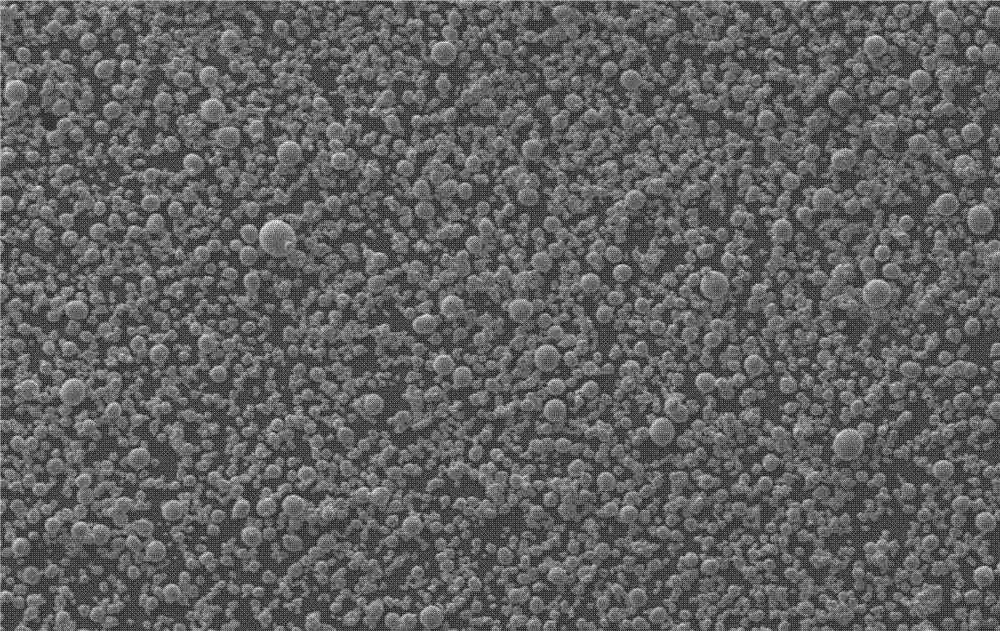
Preparation method of high-performance spherical lithium nickel cobalt aluminate anode material
A technology of lithium nickel cobalt aluminate and positive electrode materials, applied in chemical instruments and methods, battery electrodes, nickel compounds, etc., can solve problems such as uneven distribution of elements, quality differences, and deterioration of particle spherical shape, and achieve impurity The effect of low element content, good product consistency and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh 500g aluminum nitrate nonahydrate, stir and dissolve in 13kg sodium hydroxide solution; weigh 5.6kg nickel sulfate and dissolve it in 10kg distilled water; weigh 1.15kg cobalt sulfate and dissolve it in 5kg distilled water; configure 0.2mol / L acetylacetone solution; L ammonia solution; 4mol / L sodium hydroxide solution for subsequent use.
[0029] Add the above-mentioned solutions to the CSTR reactor simultaneously and concurrently, control the molar ratio of nickel-cobalt-aluminum to 0.8:0.15:0.05, and simultaneously control the molar ratio of aluminum salt solution to acetylacetone to 1:0.3, and the molar ratio of nickel-cobalt salt to ammonia water The ratio is 1:1. Under the condition of 40° C., the feeding was continued for 40 h, and the aging reaction was carried out for 10 h after the feeding was completed. After centrifuging the reaction solution, obtain a primary filter cake, add the primary filter cake to a sodium carbonate solution with a mass fraction ...
Embodiment 2
[0031] Weigh 110g aluminum hydroxide solid, stir and dissolve in 12kg sodium hydroxide solution; weigh 5.35kg nickel sulfate and dissolve it in 9.8kg distilled water; weigh 1.26kg cobalt sulfate and dissolve it in 5.3kg distilled water; configure 0.2mol / L acetylacetone solution; 5mol / L ammonia solution; 4mol / L sodium hydroxide solution for later use.
[0032] Add the above-mentioned solutions to the CSTR reactor simultaneously and concurrently, control the molar ratio of nickel-cobalt-aluminum to 0.75:0.15:0.1, and control the molar ratio of the aluminum salt solution to acetylacetone to be 1:0.4 at the same time. The molar ratio is 1:1. Under the condition of 50° C., the feeding was continued for 30 h, and the aging reaction was carried out for 10 h after the feeding was completed. After centrifuging the reaction solution, obtain a primary filter cake, add the primary filter cake to a sodium carbonate solution with a mass fraction of 3% for stirring and washing, then perform...
Embodiment 3
[0034] Weigh 1.0kg aluminum nitrate nonahydrate, stir and dissolve in 18kg sodium hydroxide solution; weigh 12.3kg nickel sulfate and dissolve it in 21kg distilled water; weigh 2.4kg cobalt sulfate and dissolve it in 10kg distilled water; configure 0.3mol / L acetylacetone solution; 10mol / L ammonia solution; 4mol / L sodium hydroxide solution for later use.
[0035] Add the above-mentioned solutions to the CSTR reactor simultaneously and concurrently, control the molar ratio of nickel-cobalt-aluminum to 0.8:0.15:0.05, and simultaneously control the molar ratio of aluminum salt solution to acetylacetone to 1:0.4, and the molar ratio of nickel-cobalt salt to ammonia water The ratio is 1:0.5. Under the condition of 40° C., the feeding was continued for 80 h, and the aging reaction was carried out for 12 h after the feeding was completed. After centrifuging the reaction solution, obtain a primary filter cake, add the primary filter cake to a sodium carbonate solution with a mass frac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com