A kind of citicoline sodium tablet and its powder direct compression preparation method
A technology for citicoline sodium and powder is applied in the pharmaceutical field, which can solve the problems of poor powder fluidity, and achieve the effects of good powder fluidity, small difference in tablet weight, and good content uniformity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
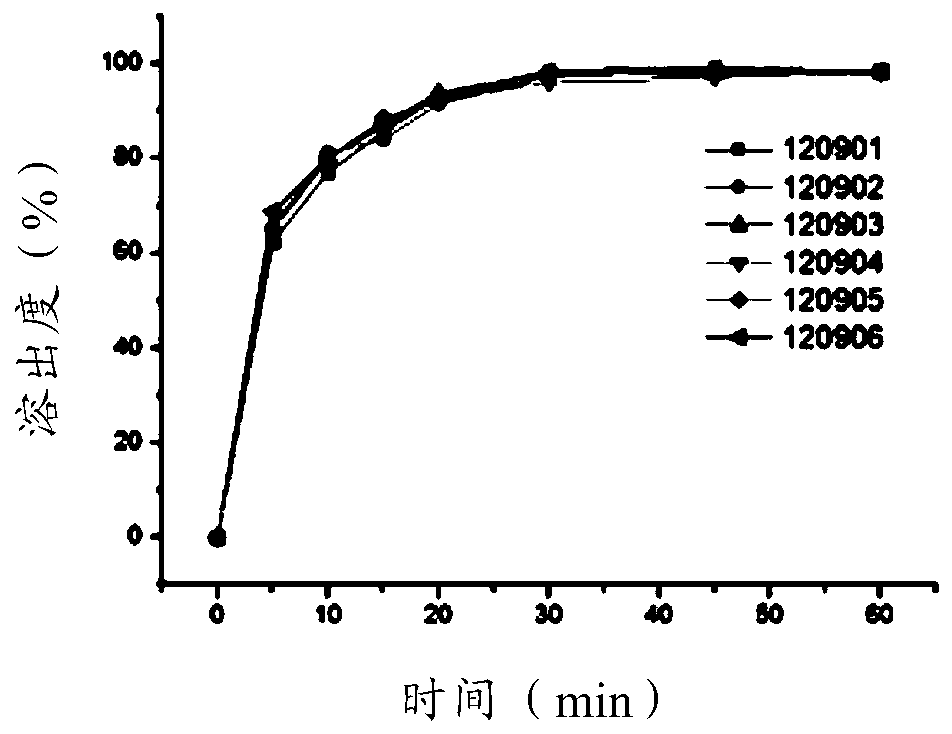
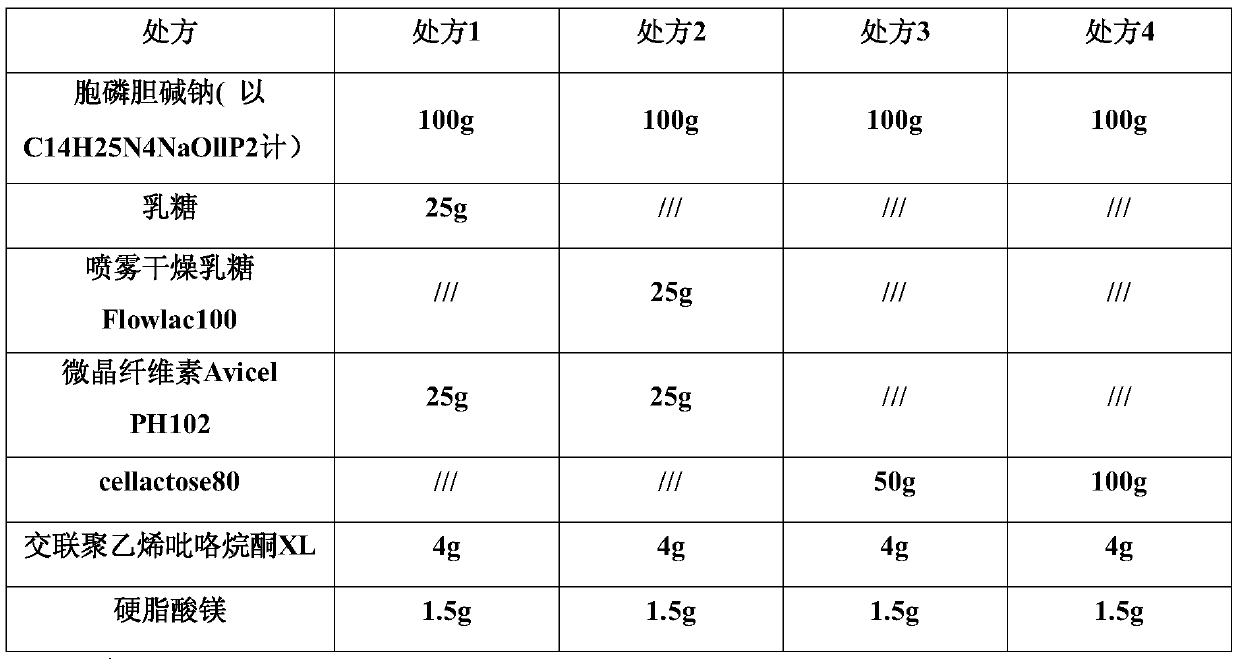
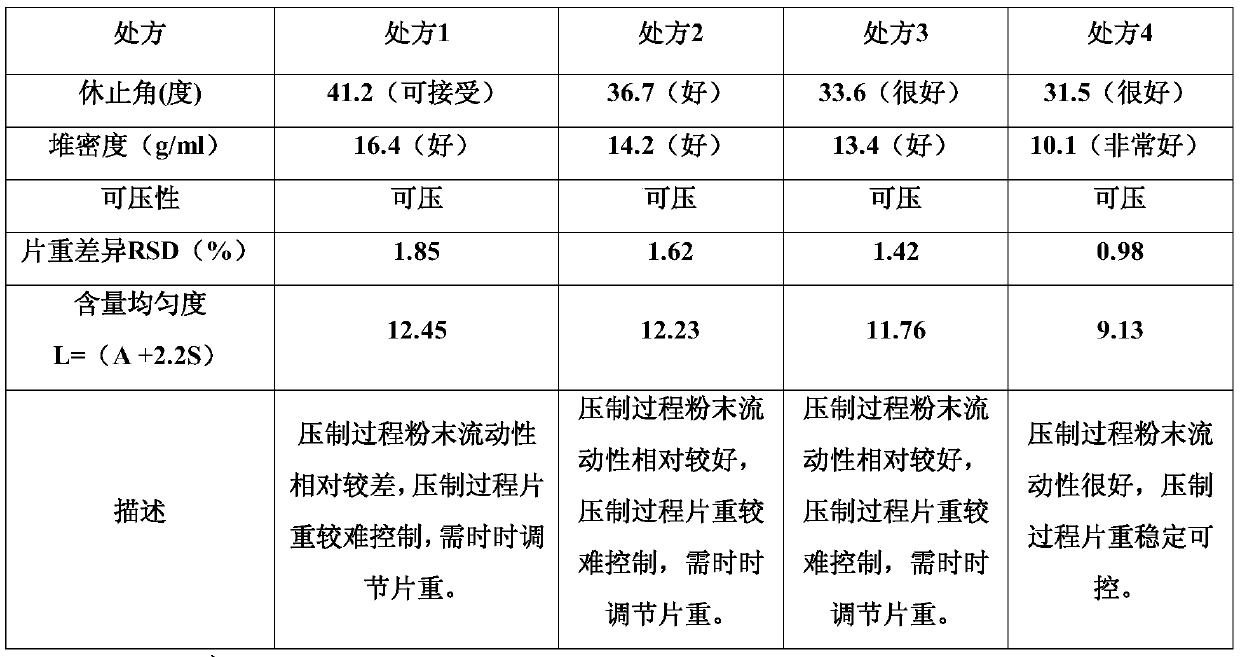
[0027] The impact of embodiment 1 different fillers and the content of fillers on citicoline sodium tablets
[0028] In this embodiment, the influence of different fillers and filler contents on citicoline sodium tablets was tested.
[0029] Table 1. The selection prescription table of different fillers and filler content of Citicoline Sodium Tablets
[0030]
[0031] Preparation Process:
[0032] 1) Take citicoline sodium and cross-linked polyvinylpyrrolidone XL to pass through a 60-mesh sieve respectively, and then take lactose, spray-dried lactose Flowlac100, microcrystalline cellulose Avicel PH102, and cellactose80 to pass through a 60-mesh sieve respectively, and set aside.
[0033] 2) Take 1 / 2 citicoline sodium and add lactose and microcrystalline cellulose Avicel PH102 in the prescription, spray-dried lactose Flowlac100 and microcrystalline cellulose Avicel PH102, or cellactose80 respectively in the blender at 20r / min, mix for 10 minutes, spare.
[0034] 3) Take...
Embodiment 2
[0046] Embodiment 2: the impact of different glidants / lubricants on citicoline sodium tablets
[0047]In this embodiment, test the influence of different glidants / lubricants on citicoline sodium tablets.
[0048] Table 3. Citicoline Sodium Tablets Glidant / Lubricant Dosage Selection Prescription Form
[0049]
[0050] Preparation Process:
[0051] 1) Take citicoline sodium and cross-linked polyvinylpyrrolidone XL to pass through a 60-mesh sieve respectively, and then take cellactose 80 to pass through a 60-mesh sieve for later use.
[0052] 2) Take 1 / 2 citicoline sodium and add micropowder silica gel and cellactose80 in the prescription to the blender at 20r / min, mix for 10 minutes, and set aside.
[0053] 3) Take the above-mixed powder, add the remaining 1 / 2 citicoline sodium, cross-linked polyvinylpyrrolidone XL and magnesium stearate to the blender at 20r / min, mix for 20 minutes, and carry out intermediate inspection.
[0054] 4) Compress the tablet with a die with a...
Embodiment 3
[0060] Embodiment 3: the impact of different disintegrants on citicoline sodium tablets
[0061] Table 5: Prescription table for selection of different disintegrants for citicoline sodium tablets
[0062]
[0063]
[0064] Preparation Process:
[0065] 1) Take citicoline sodium, cross-linked polyvinylpyrrolidone XL, sodium carboxymethyl starch, and low-substituted hydroxypropyl cellulose to pass through a 60-mesh sieve, and then take cellactose 80 to pass through a 60-mesh sieve for later use.
[0066] 2) Take 1 / 2 citicoline sodium and add micropowder silica gel and cellactose80 in the prescription to the blender at 20r / min, mix for 10 minutes, and set aside.
[0067] 3) Take the above mixed powder, add the remaining 1 / 2 citicoline sodium, cross-linked polyvinylpyrrolidone XL (or sodium carboxymethyl starch or low-substituted hydroxypropyl cellulose) and magnesium stearate to the total 20r / min in the mixer, mix for 20 minutes, and check the intermediate.
[0068] 4)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com