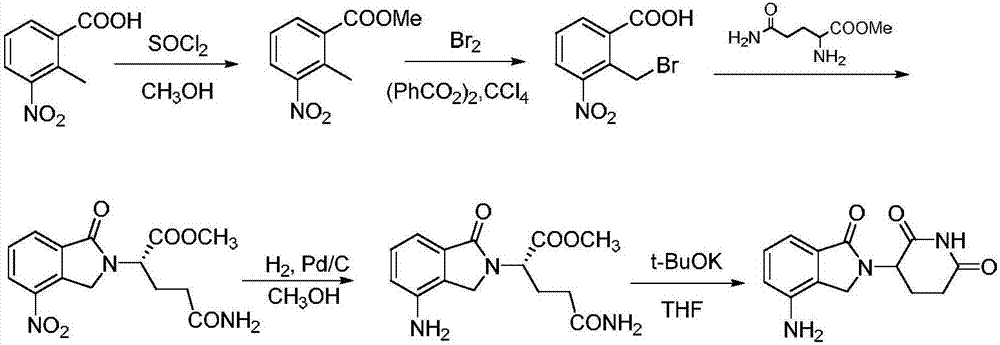
Antitumor drug lenalidomide intermediate preparation method
An anti-tumor drug, the technology of lenalidomide, applied in the direction of organic chemistry, can solve the problems of low yield, unsatisfactory preparation, complex reaction, etc., to achieve simple and easy reaction, easy to obtain raw materials, and few reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0026] Preparation of 4-nitroisoindoline
[0027] Under ice bath, add 80ml of concentrated nitric acid into 450ml of 98% concentrated sulfuric acid, stir for 10min, continue to keep the ice bath, add 50g of isoindoline under vigorous stirring, stir for 30min, continue to stir at room temperature for 8 hours, quench with ice water, and filter with suction , the filter cake is a crude product, the crude product is washed successively with water and saturated sodium bicarbonate, and recrystallized from petroleum ether to obtain 58.3g of light yellow solid 4-nitroisoindoline, the yield is 84.6%, and the HPLC purity is 99.11%; 1 HNMR (300MHz, DMSO-d 6 )δ: 7.51(d,1H), 7.43(d,1H), 7.22(t,1H), 4.04(s,2H), 3.98(s,2H), 3.47(br,1H).
Embodiment 1
[0029] Preparation of 3-(4-nitro-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione
[0030] Add 16.4g (100mmol) of 4-nitroisoindoline, 21.1g (110mmol) of 3-bromo-2,6-piperidinedione, 5.7g (30mmol) of cuprous iodide, and 53g (500mmol) of sodium carbonate In the flask, add 200ml of acetonitrile as the reaction solvent, raise the temperature to 55°C, stir and react for 5 hours, concentrate the reaction liquid, pour it into water, extract with dichloromethane, wash the organic phase with water, wash with saturated saline, dry over anhydrous sodium sulfate, and depressurize Concentrate and recrystallize from dichloromethane-n-hexane (volume ratio 1:10) to obtain 3-(4-nitro-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione 24.4g, the yield is 88.7%, the purity is 99.26%; 1 HNMR (300MHz, DMSO-d 6 )δ: 11.02(s,1H), 7.57(d,1H), 7.46(d,1H), 7.25(t,1H), 4.15(s,2H), 4.10(s,2H), 3.28(t,1H ), 2.40-2.48(m,2H), 1.91-1.95(m,2H). MS([M+H] + ): 276.11.
Embodiment 2
[0032] Preparation of 3-(4-nitro-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione
[0033] Add 16.4g (100mmol) of 4-nitroisoindoline, 23g (120mmol) of 3-bromo-2,6-piperidinedione, 7.6g (40mmol) of cuprous iodide, and 41.5g (300mmol) of potassium carbonate In the flask, add 200ml of tetrahydrofuran as a reaction solvent, raise the temperature to 60°C, stir and react for 6 hours, concentrate the reaction solution, pour it into water, extract with dichloromethane, wash the organic phase with water, wash with saturated saline, dry over anhydrous sodium sulfate, and depressurize Concentrate and recrystallize from dichloromethane-n-hexane (volume ratio 1:10) to obtain 3-(4-nitro-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione 24.9g, yield 90.4%, purity 99.44%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com