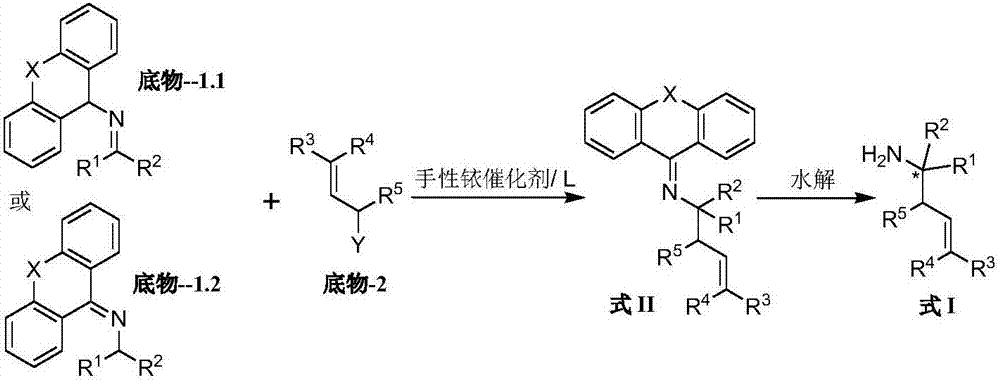
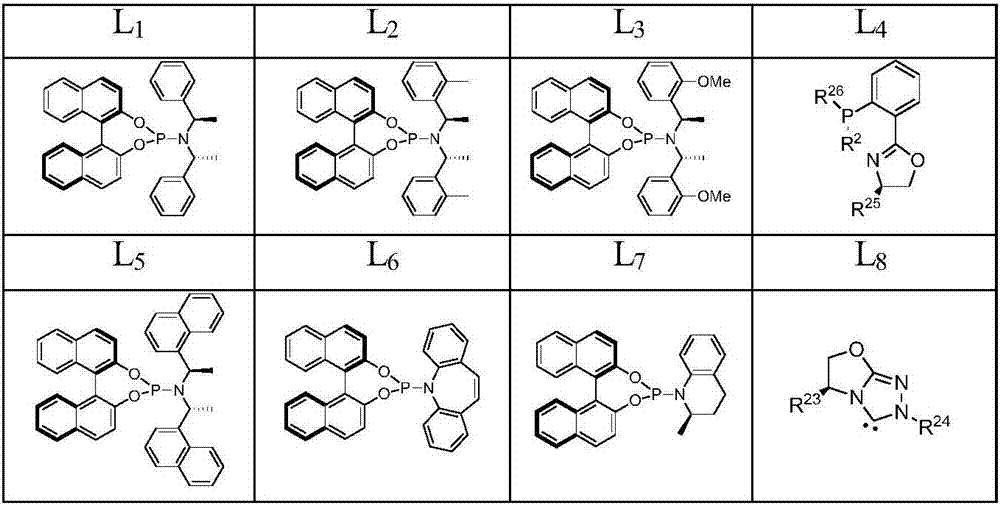
End-substituted homoallylic amine derivatives, a preparing method thereof and uses of the derivatives
A technology of high allylamine and derivatives, applied in the field of chemical medicine, can solve the problems of difficult catalysts, unable to realize allylation reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] Synthesis of Example 1 (trans)-nitrogen-benzylidene-9 hydrogen-fluorene-9-amine (substrate-1.1-1)
[0264]
[0265] 9H-fluorene-9-amine (English name: 9H-fluoren-9-amine, 1 mmol), benzaldehyde (1 mmol) and Molecular sieves (0.3 g) were stirred in dichloromethane (5 mL) at room temperature for half an hour, then filtered and the solvent removed to give Substrate-1.1-1.
Embodiment 2
[0266]Example 2 Synthesis of (trans)-nitrogen-pyridine-3-methylene-9 hydrogen-fluorene-9-amine (substrate-1.1-2)
[0267]
[0268] 9H-fluorene-9-amine (English name: 9H-fluoren-9-amine, 1 mmol), pyridine-3-formaldehyde (1 mmol) and Molecular sieves (0.3 g) were stirred in dichloromethane (5 mL) at room temperature for half an hour, then filtered and the solvent removed to give Substrate-1.1-2.
[0269] 1 H NMR (400MHz, CDCl 3 )δ:8.94(d,J=2.1Hz,1H),8.80(s,1H),8.66(dd,J=4.8,1.8Hz,1H),8.18(dt,J=7.9,2.0Hz,1H), 7.77 (dd, J=7.6, 1.0Hz, 2H), 7.47–7.37 (m, 5H), 7.31 (td, J=7.4, 1.1Hz, 2H), 5.48 (s, 1H). 13 C NMR (101MHz, CDCl 3 )δ: 160.43, 151.94, 150.68, 144.38, 141.20, 134.96, 131.78, 128.77, 127.66, 125.37, 123.83, 120.36, 74.80. HRMS (DART-TOF) C 19 h 15 N 2 + [M+H] + m / z 271.1234. Melting point: 110-120°C.
Embodiment 3
[0270] Example 3 Synthesis of (trans)-nitrogen-(4-methylthiazole-5-methylene)-9 hydrogen-fluorene-9-amine (substrate-1.1-3)
[0271]
[0272] 9H-fluorene-9-amine (English name: 9H-fluoren-9-amine, 1 mmol), 4-methylthiazole-5-aldehyde (English name: 4-methylthiazole-5-carbaldehyde, 1 mmol) ,and Molecular sieves (03 g) were stirred in dichloromethane (5 mL) at room temperature for half an hour, then filtered and the solvent removed to give Substrate-1.1-3.
[0273] IR (thin film, cm -1 ) 3016, 1619, 1448, 1260, 749. 1 H NMR (400MHz, CDCl 3 )δ:8.88(s,1H),8.72(s,1H),7.74(d,J=7.5Hz,2H),7.43(m,4H),7.31(ddd,J=7.6,7.6,1.2Hz,2H ), 5.46(s,1H), 2.67(s,3H). 13 C NMR (101MHz, CDCl 3 )δ: 155.35, 154.88, 154.01, 144.43, 141.15, 130.93, 128.72, 127.62, 125.38, 120.32, 74.61, 16.01. HRMS (DART-TOF) C 18 h 15 N 2 S + [M+H] + m / z291.0952. Melting point: 166.1~169.0℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com