Method for preparing activated pig plasma blood coagulation factor X
A blood coagulation factor and porcine plasma technology, applied in biochemical equipment and methods, enzymes, peptidases, etc., can solve the problems of low purity of porcine FXa, low purity of porcine FX, and difficulty in scaling up, and solve the problem of limited plasma sources , Improve the separation efficiency and the effect of improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Extracting and Purifying FX from Pig Plasma
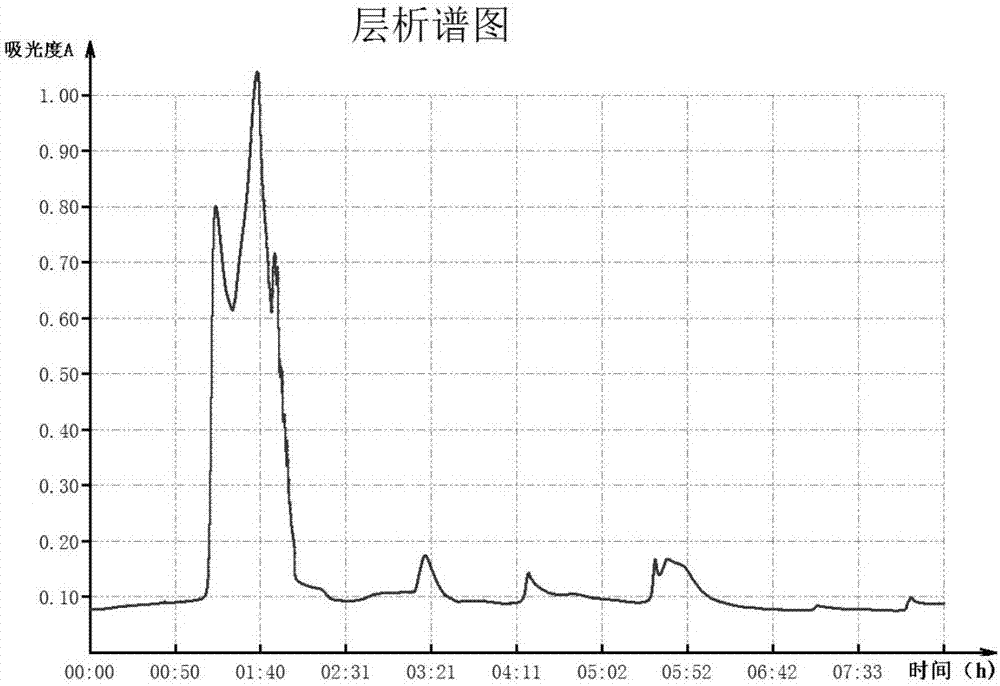
[0046] Add 80mL of 1M barium chloride solution to each liter of pig plasma, stir slowly for 30 minutes, and centrifuge to obtain the precipitate of FX crude extract. The obtained precipitate was dissolved in 0.2M EDTA pH7.4 solution, and the pH was adjusted to 7.4 before loading. Samples were loaded onto the Capto Adhere column equilibrated with 0.02M Tris-HCl buffer pH 7.4 buffer, and then unbound impurities were washed with 0.02M Tris-HCl buffer pH 7.4 buffer until the baseline was stable. 0.025M citric acid-sodium citrate buffer (pH6.5) containing 0.1M NaCl, 0.05M sodium citrate buffer (pH5.8) containing 0.15M NaCl, 0.05M citric acid-sodium citrate buffer ( pH3.0) stage elution, elution speed 1.0mL / min, 5min / tube, automatically collect eluate. Monitor with a nucleic acid and protein detector, according to A 280nm Values plot the elution line, such as figure 1 shown. Use the chromogenic substrate method to...
Embodiment 2
[0047] Example 2 Plasma directly prepares FX through the chromatographic column
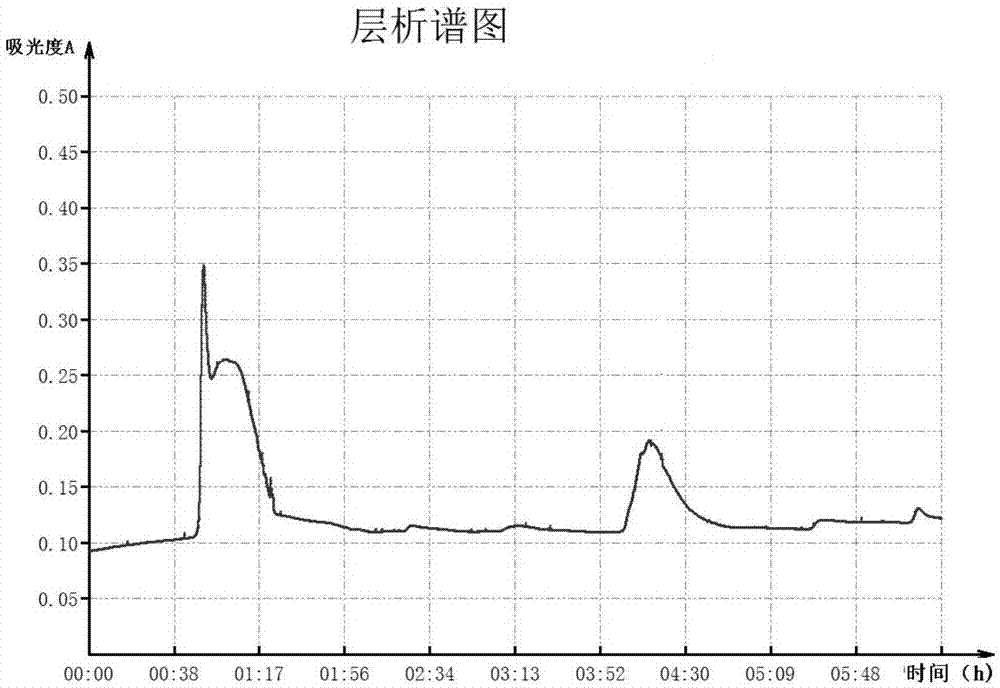
[0048] Add an appropriate amount of PPA HyperCel filler that has been equilibrated with an equilibration buffer (0.02M Tris-HCl buffer pH 7.4) into the porcine plasma, and stir gently for 1 hour. Pack the protein-adsorbed filler into the column, and then fully wash the unbound foreign protein with the equilibration buffer, about 10 times the column bed volume of the equilibration buffer is required. 0.025M citric acid-sodium citrate buffer (pH6.5) containing 0.1M NaCl, 0.05M citric acid-sodium citrate buffer (pH5.8) containing 0.15M NaCl, 0.05M citric acid-sodium citrate buffer Buffer (pH3.0) stage elution, each elution stage requires about 3 times the column bed volume of elution buffer, and collect the eluate. The chromogenic substrate method was used to detect the activity of each collected part, and the eluate with FX activity was determined, and the collected part was dialyzed and concentra...
Embodiment 3
[0049] Example 3 activates FX
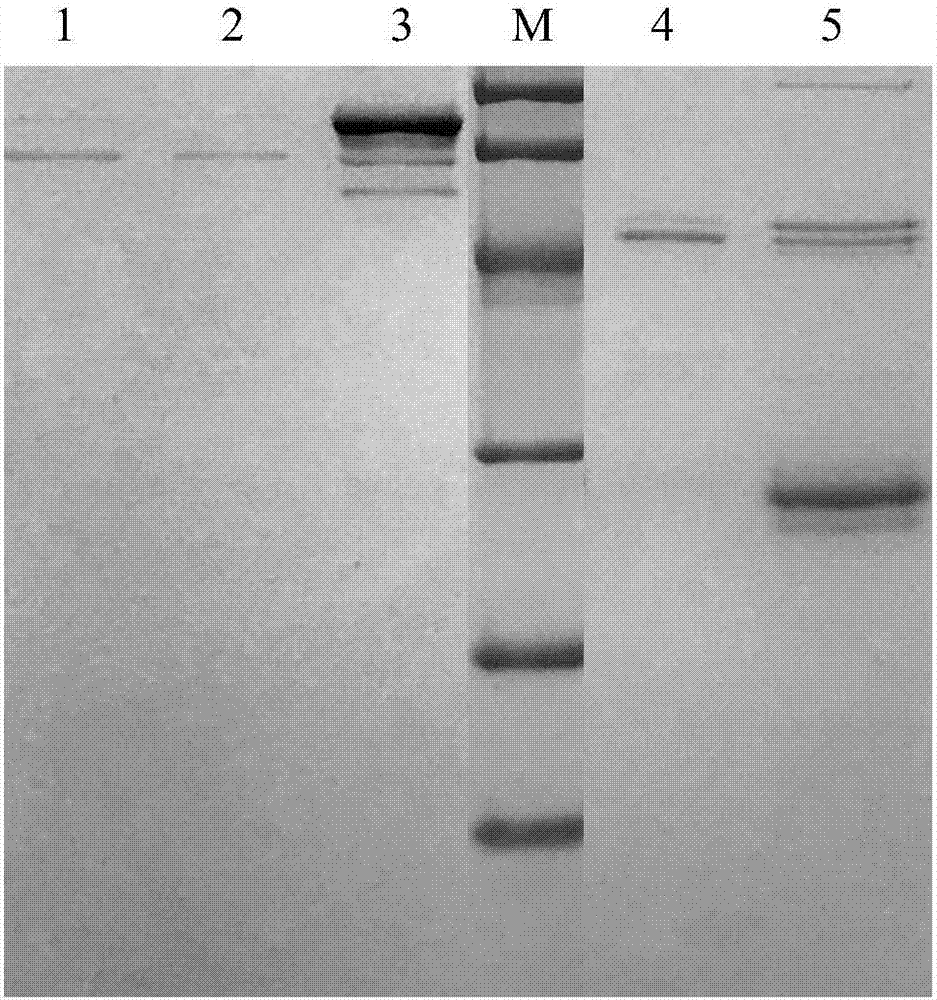
[0050] Collect the eluate with FX activity in Example 1 or Example 2 and add sodium citrate solid to make the final concentration 0.35g / ml, and activate at room temperature for 23 hours. Then add purified RVV-X freeze-dried powder to it to make the concentration 0.5mg / ml, then add 100mL 0.2M calcium chloride solution and 5mL rabbit brain phospholipid to each liter of eluent, activate at 37°C for 3 hours . After activation is complete, the concentrated sample is dialyzed and immediately isolated and purified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com