Recombinant expression adenovirus cilia protein peptide, adenovirus subunit vaccine and preparation method of recombinant expression adenovirus cilia protein peptide
A subunit vaccine, ciliated protein technology, applied in antiviral immunoglobulins, viral peptides, botanical equipment and methods, etc., can solve the problem of unreported cross-neutralization, achieve convenient production and purification, and high expression , strong immunogenic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
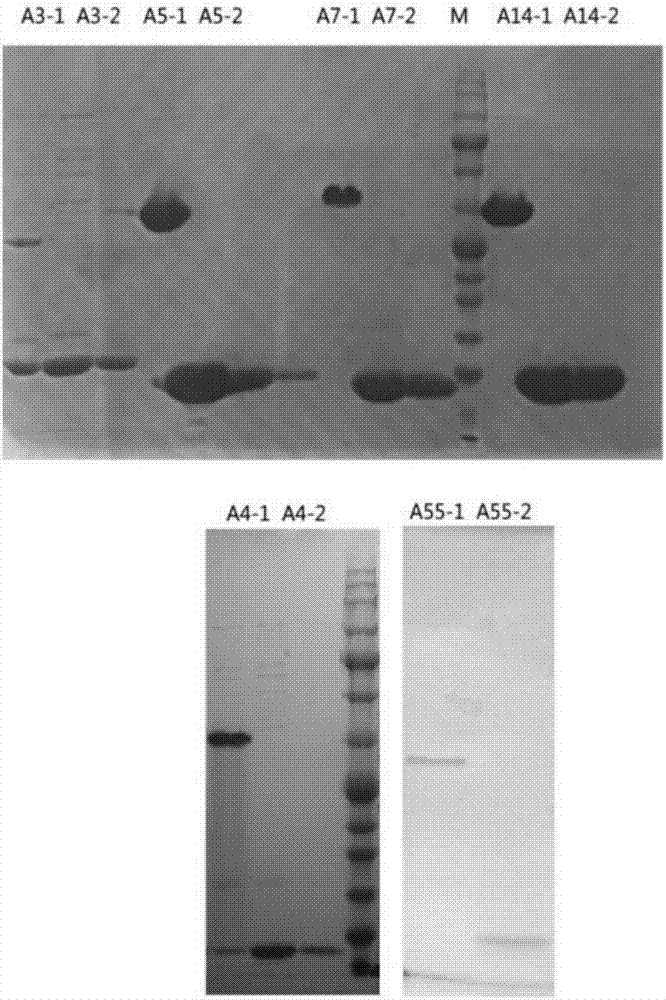
[0039] Embodiment 1 Expression of recombinant human adenovirus knob polypeptide
[0040] 1. Escherichia coli preferred codons were optimized for human type 3, type 7, type 14, type 4, and type 5 adenovirus knob genes to adapt to expression in E. coli, and the genes were synthesized separately and cloned into the prokaryotic expression vector PQE30 (Qiagen Company) middle;
[0041] 2. Using the human adenovirus type 55 genome as a template, PCR was performed with the primer pair A55Kn-RH / A55Kn-UB to amplify the type 55 knob gene, and the PCR fragment was double-digested with BamHI and HindIII and connected to the above-mentioned PQE30 to transform Escherichia coli Top10 chemically competent, pick a single colony for gene sequencing. Take colonies consistent with the theoretical sequence without mutations and extract plasmids;
[0042] A55Kn-RH: AGCTAATTAAGCttagtcgtcttctctgatgtagta (SEQ ID No: 13)
[0043] A55Kn-UB:ACCATCACGGATCCaacaacatttgcattgatgac (SEQ ID No: 14)
[0044]...
Embodiment 2
[0053] The immunogenicity test of embodiment 2 recombinant protein
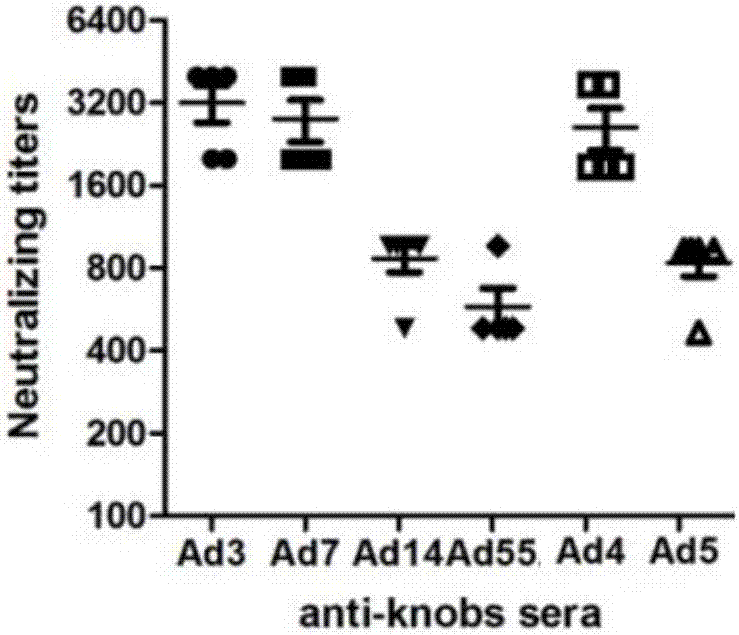
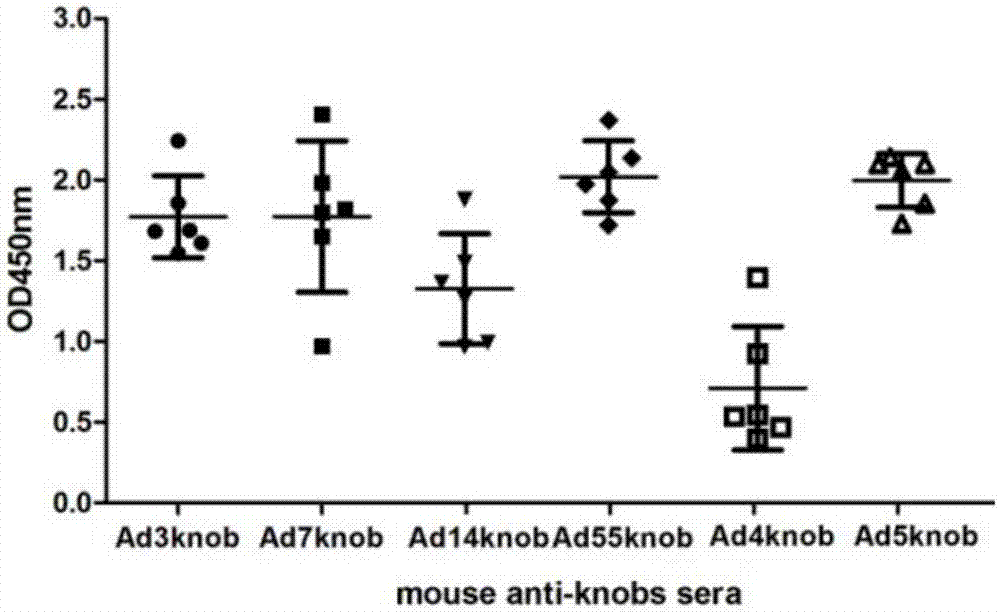
[0054] In order to analyze the immunogenicity of the recombinant protein of Example 1, the recombinant protein was added to the emulsified aluminum phosphate adjuvant (ADJU-PHOS, Brenntag Biosector) to immunize mice for four times, and each mouse was immunized with 40ug protein each time. Serum was collected 7 days after the last immunization for ELISA detection and cell microneutralization experiments.
[0055] The result is as figure 2 and image 3 As shown, the results show that all types of recombinant knob immunized mice can induce high-titer antibody responses, and the neutralizing antibody titers to respective types of adenoviruses are about 500-4000 ( figure 2 ), the titer of ELISA detection with recombinant protein antigen can reach 10 6 -10 7 ( image 3 ).
Embodiment 3
[0056] Example 3 Mouse Anti-Adenovirus Ciliary Protein Head (knob) Serum Analysis of Cross-Neutralization of Different Types of Adenoviruses
[0057] The anti-ciliated protein head serum of Example 2 after four times of immunization was collected for cross-neutralization detection, and the results are shown in Table 1.
[0058] Table 1 The cross-neutralization effect of mouse anti-adenovirus ciliary protein head serum on different types of adenovirus
[0059]
[0060]
[0061] -: No neutralization detected
[0062] The results in Table 1 show that some types have cross-neutralizing effects on multiple types of adenoviruses in the same group, and this cross-neutralizing effect is very strong. Ad3 Knob antiserum can neutralize human adenovirus types 3, 14, and 55, so Ad3 Knob can be used as a multivalent adenovirus vaccine candidate for preventing human adenovirus types 3, 14, and 55.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com