Method for purifying antibody and buffer solution used therein
A buffer and antibody technology, applied in the field of biomedicine, can solve problems such as side effects and unsatisfactory effects, and achieve the effect of improving purification yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 、 Nivolumab ( IgG4 Subtype antibody representative) Preparation of antibody stock solution
[0038] The light chain and heavy chain genes of Nivolumab were cloned, inserted into the pcDNA3.1 expression vector, transfected into CHO-K1 expression host cells, and screened for high-expression clones. Carry out mass culture and induce expression under CO2 conditions. After 2 weeks of culture, collect the cell culture medium, and use a deep filter system (Pall Company) for clarification and filtration. First, use a deep filter with a pore size of 0.6-9 μm to remove cells, cell debris and insoluble substances. , and then use a depth filter with a pore size below 0.1 μm to remove fine particles, and collect the filtrate as the antibody stock solution for studying the Nivolumab purification process. The protein content in the antibody stock solution was determined to be 1.6 mg / ml by OD280 ultraviolet absorption method, and the pH value was determined to be 7...
Embodiment 2
[0039] Example 2 , using conventional techniques for Nivolumab antibody stock solution Protein A affinity chromatography and low pH Elution
[0040] ① ProteinA affinity chromatography and low pH Elution
[0041] Using GE’s rProteinA Sepharose 4 Fast Flow purification medium and Avant 150 protein purification system, the Nivolumab antibody stock solution was loaded at a dose of 30 mg protein / ml medium, and the binding buffer (Binding Buffer) was 20 mM phosphate buffer at pH 7.0 , the elution buffer (Elution Buffer) was 25mM citrate buffer with pH 3.5, the flow rate was 5ml / min, and the eluate containing protein was collected.
[0042] ②Ultra-high performance liquid chromatography ( UPLC ) combined with size exclusion chromatography ( SEC ) to analyze the eluate
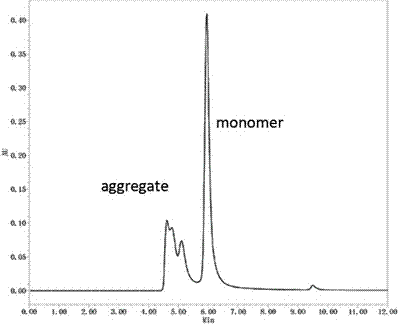
[0043] The pH value of the eluate was determined to be 3.9, and the eluate containing the target protein (i.e. Nivolumab antibody) was sampled, using UPLC H-Class Bio ultra-high per...
Embodiment 3
[0044] Example 3 、Use ion exchange chromatography to separate and identify target proteins and aggregates in affinity chromatography eluates
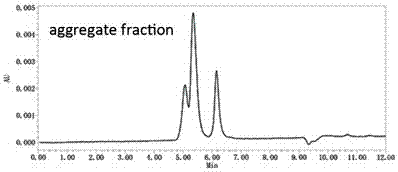
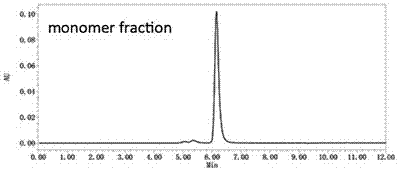
[0045] Fractogel® EMD SO3 (S) strong ion exchange chromatography (Merck KGaA company) was used to separate the aggregates and target proteins in the affinity chromatography eluate in Example 2, and the equilibrium buffer was 20 mM vinegar at pH 5.0 Salt buffer, the elution buffer is 20 mM acetate buffer at pH 5.0 containing 1M sodium chloride, the UV monitoring of the protein purification instrument shows that there are 2 protein elution peaks, and the 2 elution peaks are collected The liquid was analyzed by ultra-high performance liquid chromatography combined with molecular size exclusion chromatography in Example 2, and the first elution peak was the elution peak of Nivolumab monomer (Monomer), containing only 1% of aggregates, and the second The elution peak is the elution peak of aggregates, containing 70% aggregates and 30% N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com