Medicine for curing diarrhea and its preparing process
A technology of drugs and compositions, applied in the fields of biotechnology and medicine, which can solve the problems of low ITF content, difficulty in structure and function research, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
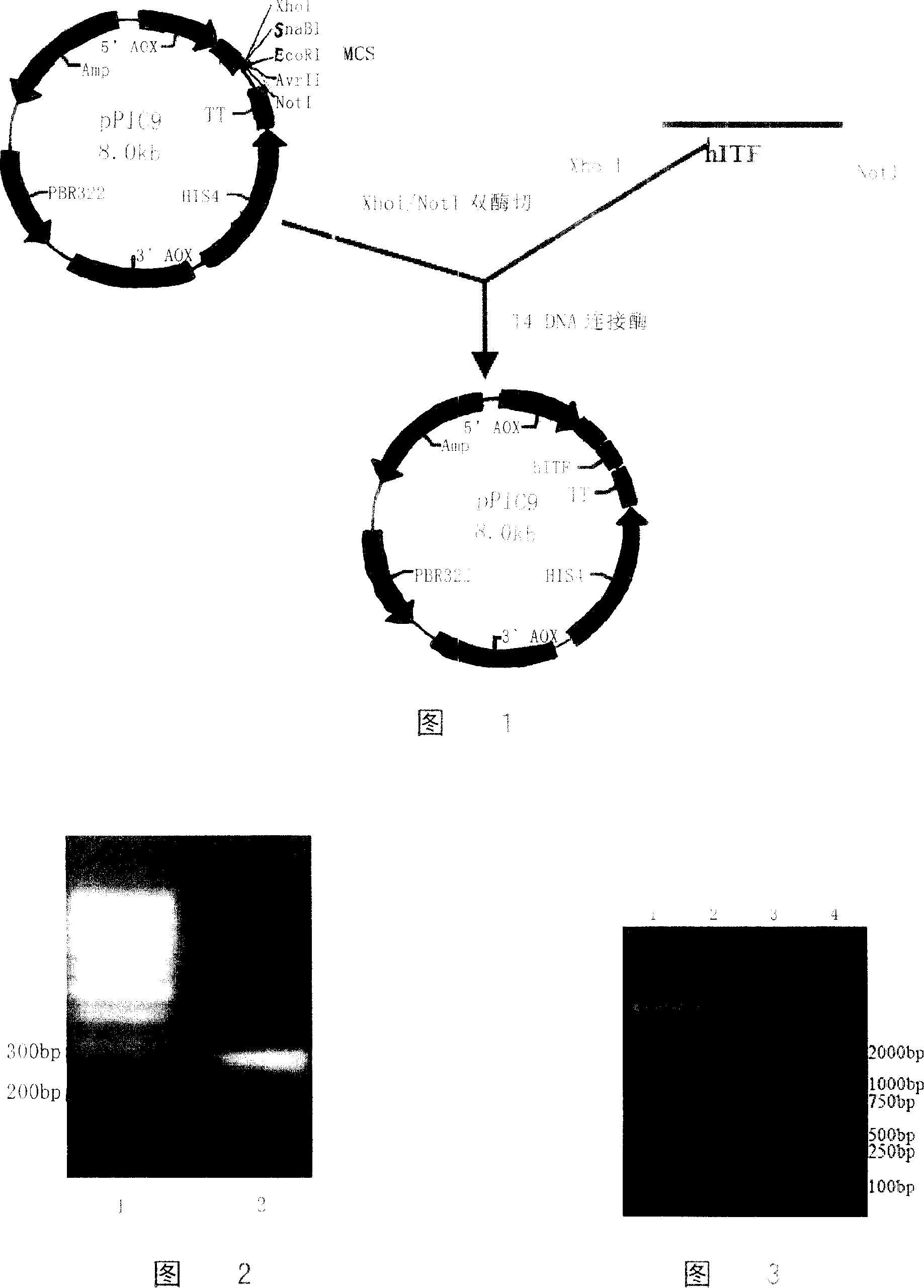
[0111] Embodiment 1 plasmid construction
[0112] Plasmids and strains: Plasmid pPIC9 is a product of Invitrogen; strain TG1 is a product of Sigma.
[0113] Enzymes and reagents: Restriction enzymes (XhoI, NotI) and T4 DNA ligase are products of TaKaRa; total cell RNA extraction kit Trizol, reverse transcription reagents and PCR reagents are all products of GIBCO BRL; DNA gel The recovery kit and plasmid purification kit were products of Shanghai Huashun Bioengineering Co., Ltd.; pfu polymerase was purchased from Shanghai Sangon Bioengineering Company; primers were synthesized and purified by Shanghai Sangon Bioengineering Company; DNA Marker was GeneRuler 100bp DNA Ladder and DL 2000. Other reagents were imported or domestic analytically pure.
[0114] 1. Extraction of total RNA from human colonic mucosa
[0115] The intestinal mucosa was biopsied by colonoscopy and immediately put into liquid nitrogen for storage at -80°C. Colonic mucosa was placed in a homogenizer, and ...
Embodiment 2
[0133] Expression and purification of embodiment 2 protein
[0134] 1. Recombinant plasmid transformation
[0135] Extract about 10ug of the recombinant plasmid, digest it with SalI, ethanol precipitate and recover the linearized plasmid, and transform it into GS115 (Mut + his4) In yeast competent cells, the electric shock products were spread on MD plate (13.4g / LYNB, 20g / L glucose, 4×10 -4 g / L biotin, 15g / L agar), cultured at 30°C for 2-3 days, and observed the growth of transformants.
[0136] 2. Identification and screening of transformants
[0137] According to the operation manual provided by Invitrogen, select universal primers 5'AOX1 and 3'AOX1, and amplify the target fragment by colony PCR to screen positive transformants. Add 30 cycles.
[0138] 5'AOX1: 5'-GACTGGTTCCAATTGACAAGC-3' (SEQ ID NO: 3);
[0139] 3'AOX1: 5'-GCAAATGGCATTCTGACATCC-3' (SEQ ID NO: 4).
[0140] Pick a single colony from the MD plate into the YPD medium (10g / L yeast extract, 20g / L peptone, 1...
Embodiment 3
[0179] Example 3 Identification of biological activity of hITF
[0180] 6.1 Method
[0181] Rat small intestinal epithelial cells (IEC-6, purchased from the Cell Collection Center of Peking Union Medical College, China) and a cell migration kit (Corning, US) were used to observe the effect of different concentrations of recombinant ITF on cell migration. Cells were cultured in serum-free medium for 24 hours to cause cell starvation, and then digested with trypsin to prepare cell suspension with a cell concentration of 0.5-1×10 6 / ml, add 300ul of cell suspension to the end of each built-in well plate of the 24-well plate (the bottom is a membrane with a pore size of 0.8um, and epithelial cells can pass through the well), and add physiological saline or 10 -9 M, 10 -7 M and 10 -5 For the recombinant ITF of M, add 500ul medium containing 5% fetal bovine serum to the outside of the built-in well plate. After culturing for 14 hours, suck out the cell suspension in the built-in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com