Hemostasis composition with stable drug effect
A composition and efficacy technology, applied in the field of hemostatic compositions, can solve the problems of enhanced inflammatory response, difficult absorption, delayed wound healing, etc., and achieve the effects of degrading absorption, strong biocompatibility, and promoting wound healing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 (preparation one)
[0024] Choose: 10 parts by weight of starch solution, starch solution comprises the composition of pullulan, carboxymethyl starch and distilled water, starch solution comprises the composition of the pullulan of 20 parts by weight, the carboxymethyl starch of 10 parts by weight and the distilled water of 300 parts by weight ;
[0025] 5 parts by weight of coagulation factor solution, the coagulation factor solution is a mixed solution of activated coagulation factor VII (FAIIa) and coagulation factor X (FX), and the coagulation factor solution includes 1-20 μm of FAIIa and 5-400 μm of FX;
[0026] 5 parts by weight of blood coagulation amino acid solution, the blood coagulation amino acid solution is ε-polylysine solution, and the blood coagulation amino acid solution includes 40 parts by weight of ε-polylysine;
[0027] Chitosan 10 weight parts, chitosan comprises the chitosan dilute solution of weight part 35;
[0028] 5 parts by wei...
Embodiment 2
[0033] Embodiment 2 (preparation two)
[0034] Select: 20 parts by weight of starch solution, starch solution comprises amylopectin, carboxymethyl starch and distilled water, starch solution comprises 20 parts by weight of amylopectin, carboxymethyl starch of 10 parts by weight and distilled water of 300 parts by weight ;
[0035] 7 parts by weight of coagulation factor solution, the coagulation factor solution is a mixed solution of activated coagulation factor VII (FAIIa) and coagulation factor X (FX), and the coagulation factor solution includes FAIIa of 1-20 μm and FX of 5-400 μm;
[0036] 6 parts by weight of blood coagulation amino acid solution, the blood coagulation amino acid solution is ε-polylysine solution, and the blood coagulation amino acid solution includes 40 parts by weight of ε-polylysine;
[0037] Chitosan 15 weight parts, chitosan comprises the chitosan dilute solution of weight part 35;
[0038] 8 parts by weight of the water-soluble polymer auxiliary m...
Embodiment 3
[0043] Embodiment 3 (biological experiment)
[0044] Experiment preparation
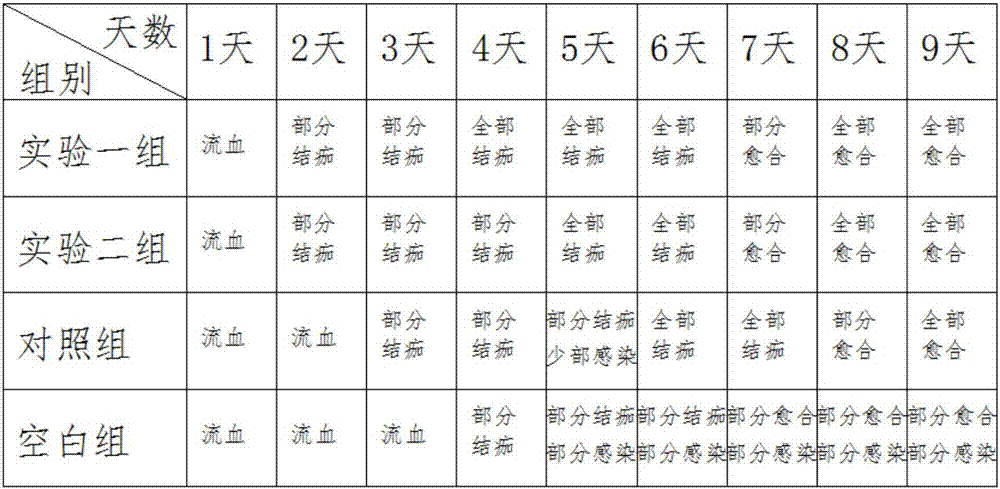
[0045] First, forty mice were prepared and fed for ten days under the same conditions to prepare the hemostatic compositions in Example 1 and Example 2, which were respectively experimental group 1 and experimental group 2, and then selected common hemostatic compositions on the market, Mark the control group, allocate ten mice to each of the above three groups, and the remaining ten mice are the blank control group.
[0046] conduct experiment
[0047] First, the mice were fed under the same conditions, and then the mice were scratched at the same position on the back, with the same wound size and length;
[0048] The mice in the experimental group, the experimental group 2 and the control group applied medicine to the wound in the morning and evening respectively, with the same amount of medicine applied;
[0049] Observation records, recorded once a day, the records are as follows:
[0050] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com