A kind of hemostatic material based on the combination of chitosan and tissue factor and its preparation method
A tissue factor and hemostatic material technology, applied in surgical adhesives, bandages, surgery, etc., can solve the problems of affecting wound healing, limited hemostasis effect, skin burns, etc., and achieve good hemostasis effect, stable and rapid hemostasis effect, and short hemostasis time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
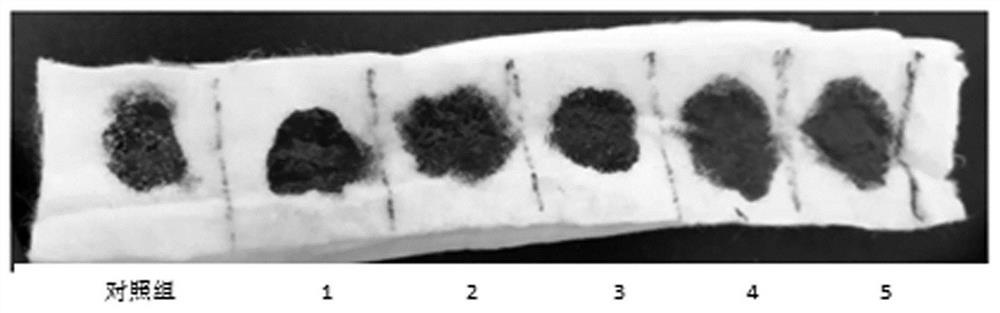
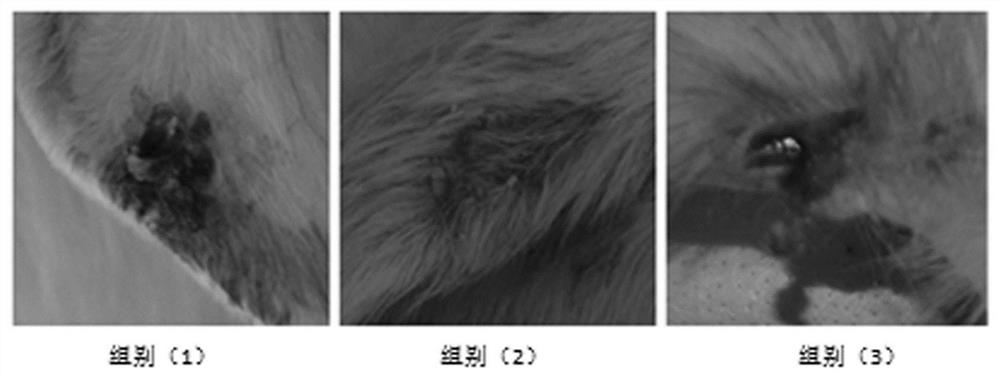
Examples
Embodiment 1
[0024] Embodiment 1: a kind of preparation method based on chitosan and tissue factor combined hemostatic material of the present invention is:
[0025] (1) Weigh 1.2g of phosphatidylcholine, 0.8g of phosphatidylserine, 5g of trehalose, 2.5g of calcium chloride, and 0.005g of human recombinant tissue factor protein, and prepare 100ml of a mixed solution with water, mix well, and use high pressure to uniformly Emulsify with a quality machine and extruder, operating pressure 600bar, temperature 10°C-20°C, high-pressure homogenization, add 200nm filter membrane to extrude, repeat 10 times, and form an emulsion, which is the human recombinant tissue factor emulsion. The average particle size of the lipid droplet particles is between 100-200 nanometers.
[0026] (2) Weigh 5,000 grams of carboxymethyl chitosan, add it to 25 liters of water, slowly add 100 ml of the emulsion in step 1, stir and mix well, and control the temperature at 15°C-20°C.
[0027](3) Drying: Pre-freeze the mi...
Embodiment 2
[0029] Embodiment 2: a kind of preparation method based on chitosan and tissue factor combined hemostatic material is:
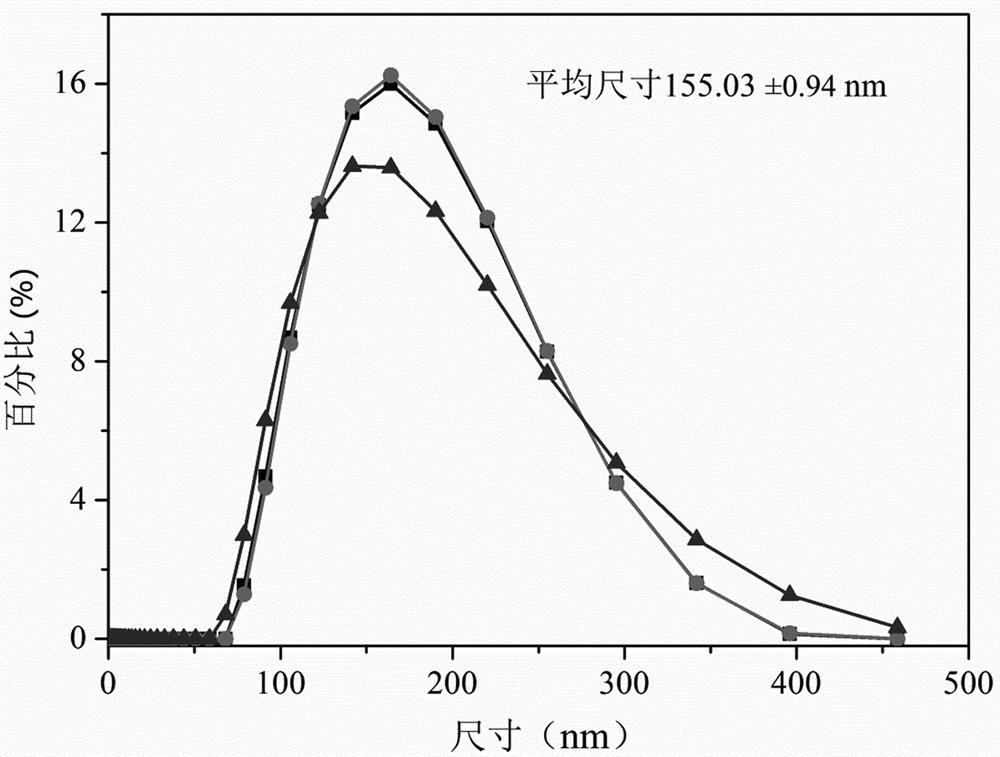
[0030] (1) Weigh 2.1g of phosphatidylcholine, 0.9g of phosphatidylserine, 7.5g of trehalose, 3g of calcium chloride, 0.005g of human recombinant tissue factor protein, and prepare 100ml of mixed solution with water, mix well, and use high pressure to Add extruder to emulsify, homogenize under high pressure at temperature 10-20°C and pressure 600bar, extruder add 200nm filter membrane to extrude, repeat 10 times to form emulsion, which is human recombinant tissue factor emulsion. The average particle size of lipid droplet particles is about 155 nanometers.
[0031] (2) Weigh 5,000 grams of carboxymethyl chitosan, add it to 25 liters of water, slowly add 100 ml of the emulsion in step 1, stir and mix well, and control the temperature at 15°C-20°C.
[0032] (3) Drying: pre-freeze the mixture in step 2 at -80°C for 10 hours, then put it into a freeze-drying box...
Embodiment 3
[0034] Embodiment 3: a kind of preparation method based on chitosan and tissue factor combination hemostatic material is:
[0035] (1) Weigh 2.1g of phosphatidylcholine, 0.9g of phosphatidylserine, 7.5g of trehalose, 3g of calcium chloride, 0.01g of human recombinant tissue factor protein, and prepare 100ml of mixed solution with water, mix well, and use high pressure to Adding extruder to emulsification, high-pressure homogenization at operating temperature of 10-20°C and pressure of 650bar, extruding with 200nm filter membrane in extruder, repeated 8 times to form emulsion, which is human recombinant tissue factor emulsification liquid. The average particle size of the lipid droplet particles is between 100-200 nanometers.
[0036] (2) Weigh 5,000 grams of carboxymethyl chitosan, add it to 25 liters of water, slowly add 100 ml of the emulsion in step 1, stir and mix well, and control the temperature at 15°C-20°C.
[0037] (3) Drying: pre-freeze the mixture in step 2 at -80...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com