Medicinal preparation containing cetilistat and preparation method thereof
A pharmaceutical preparation, the technology of the new lixistat, applied in the field of medicine, can solve the problems of poor stability and slow disintegration of samples, and achieve the effects of good stability, excellent disintegration speed and excellent hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
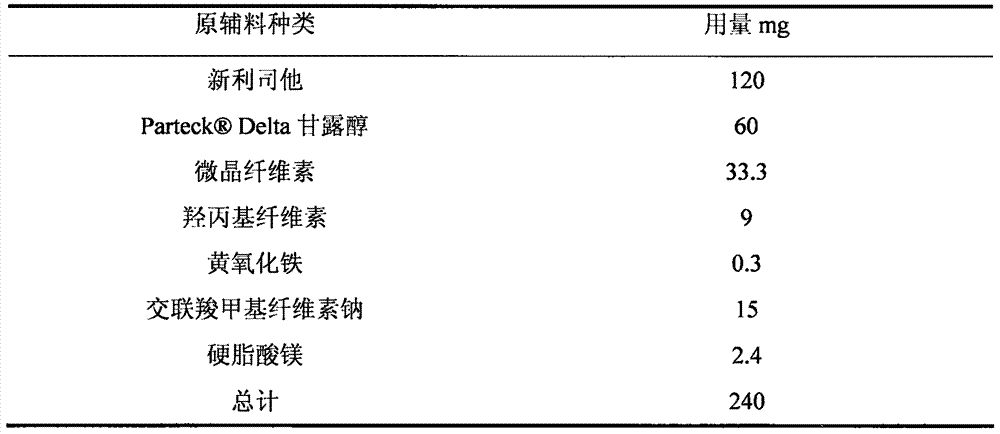
[0028] Tablets containing Neolistat were prepared at the composition ratios shown in Table 1 as follows.
[0029] Briefly, Neolistat, mannitol and microcrystalline cellulose were placed in a fluid bed dry granulator, and the mixture was preheated and mixed. The mixture was granulated while spraying an aqueous solution of hydroxypropyl cellulose and yellow iron oxide to obtain a granulated powder. The obtained granulated powder was passed through a 16-mesh sieve to obtain a sieved powder. To the sieved powder was added croscarmellose sodium and magnesium stearate, and the mixture was blended in a bag to obtain a blended powder. Using a rotary tablet press, using a 14×8 mm punch, the mixed powder was tableted, each tablet 240 mg.
[0030] Table 1 prescription composition
[0031]
Embodiment 2
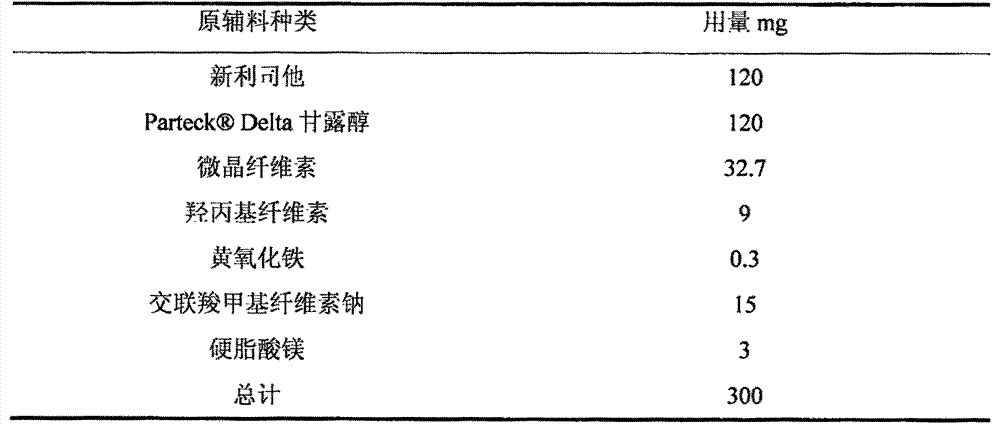
[0033] Tablets containing Neolistat were prepared at the composition ratios shown in Table 2 as follows.
[0034] Briefly, Neolistat, mannitol and microcrystalline cellulose were placed in a fluid bed dry granulator, and the mixture was preheated and mixed. The mixture was granulated while spraying an aqueous solution of hydroxypropyl cellulose and yellow iron oxide to obtain a granulated powder. The obtained granulated powder was passed through a 16-mesh sieve to obtain a sieved powder. To the sieved powder was added croscarmellose sodium and magnesium stearate, and the mixture was blended in a bag to obtain a blended powder. Using a rotary tablet press, using a 14×8 mm punch, the mixed powder was tableted, each tablet 300 mg.
[0035] Table 2 Prescription Composition
[0036]
Embodiment 3
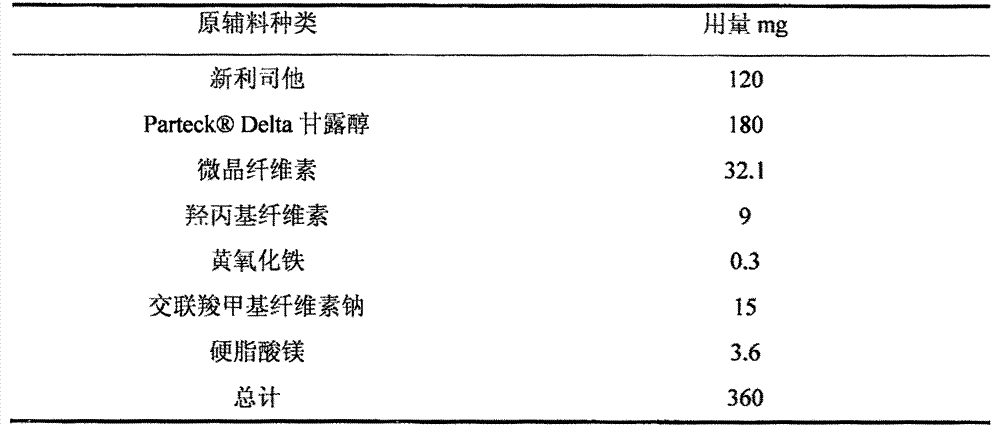
[0038] Tablets containing Neolistat were prepared at the composition ratios shown in Table 3 as follows.
[0039] Preparation process: Briefly, neolistat, mannitol and microcrystalline cellulose were placed in a fluidized bed dry granulator, and the mixture was preheated and mixed. The mixture was granulated while spraying an aqueous solution of hydroxypropyl cellulose and yellow iron oxide to obtain a granulated powder. The obtained granulated powder was passed through a 16-mesh sieve to obtain a sieved powder. To the sieved powder, croscarmellose sodium and magnesium fecal acid were added, and the mixture was mixed in a bag to obtain a mixed powder. Using a rotary tablet press, using a 14×8 mm punch, the mixed powder was tableted, each tablet 360 mg.
[0040] Table 3 prescription composition
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com