Method for synthesizing M(BH4)n and/or M2/nB12H12
A technology of nb12h12 and B10H14, which is applied in the field of inorganic material synthesis process, can solve the problems of increased risk and complexity, and achieve the effects of high safety, environmental protection and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] In a pure Ar protected glove box, use commercial NaNH 2 and B 10 h 14 As the initial reactant, NaNH 2 and B 10 h 14 Grind in a mortar and mortar for 30 minutes at a molar ratio of 5:1 until well mixed.
[0034] Take about 0.15g of a well-mixed sample and place it in a volume of 0.7cm 3 Inside the stainless steel reaction crucible, sealed. The stainless steel reaction crucible after sealing the sample was heated in a muffle furnace under the following conditions: 300°C for 1 hour; 300°C for 5 hours; 300°C for 10 hours; 400°C for 10 hours. After the temperature dropped to room temperature, the reaction crucible was taken out, opened in an Ar protection glove box, and the sample was recovered.
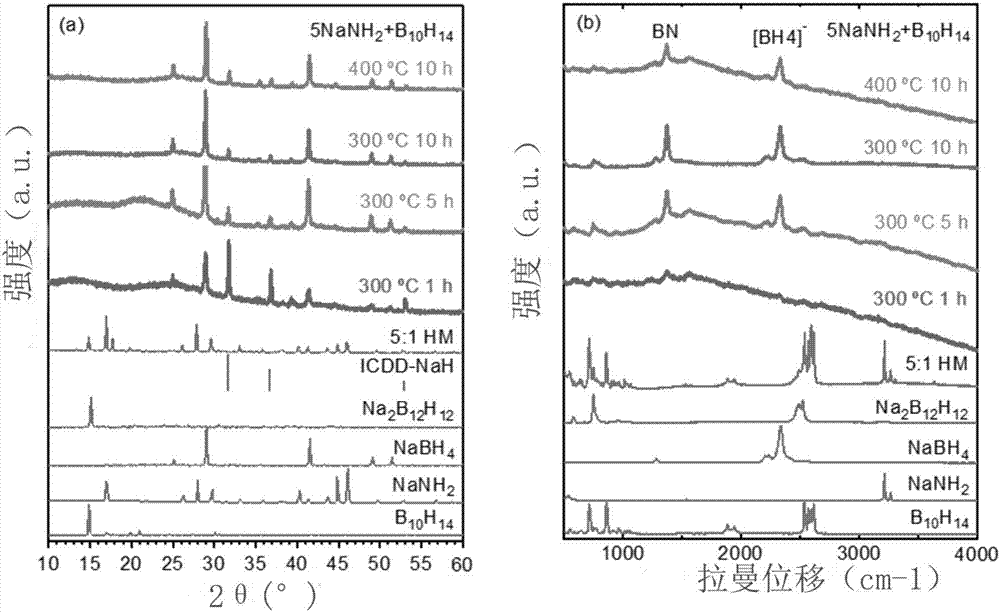
[0035] figure 1 It is the XRD and Raman diagram of the reaction product, and a single NaBH is obtained 4 .
[0036] XRD and Raman two detection methods for NaBH 4 and Na 2 B 12 h 12 Sensitivity is different, XRD is easier to detect NaBH 4 , Raman is easier to detect ...
Embodiment 2
[0039] In a pure Ar protected glove box, use commercial NaNH 2 and B 10 h 14 As the initial reactant, NaNH 2 and B 10 h 14 Grind in a mortar and mortar for 30 minutes at a molar ratio of 3.5:1 until well mixed.
[0040] Take about 0.15g of a well-mixed sample and place it in a volume of 0.7cm 3 Inside the stainless steel reaction crucible, sealed. The stainless steel reaction crucible after sealing the sample was heated in a muffle furnace under the following conditions: 100°C for 10 hours; 200°C for 10 hours; 300°C for 10 hours; 400°C for 10 hours. After the temperature dropped to room temperature, the reaction crucible was taken out, opened in an Ar protection glove box, and the sample was recovered.
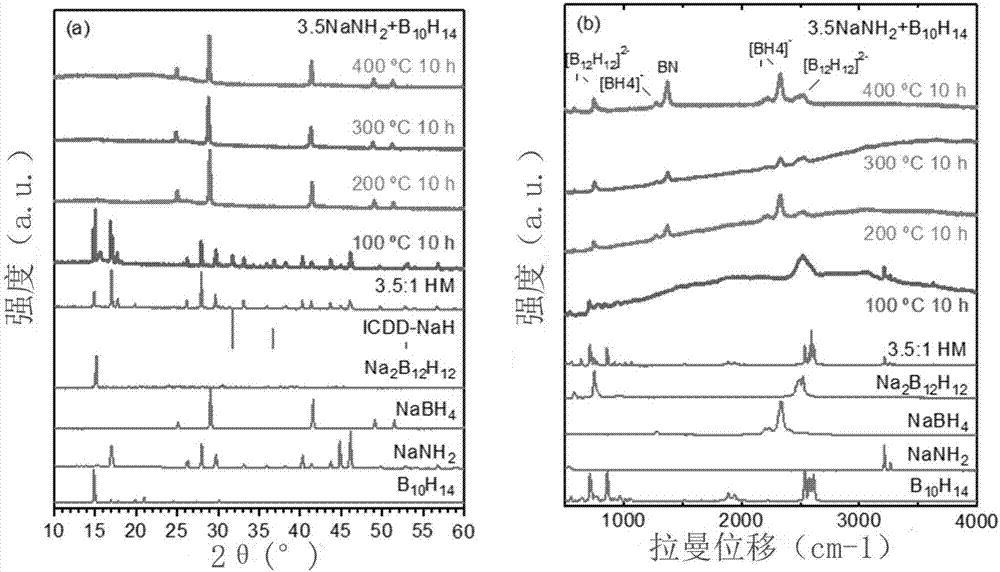
[0041] figure 2 is the XRD and Raman diagram of the reaction product, NaBH 4 more than Na 2 B 12 h 12 , a weaker Na can be seen in the Raman spectrum 2 B 12 h 12 peak. In addition to the ratio of raw materials, the reaction conditions can also significantly af...
Embodiment 3
[0043] In a pure Ar protected glove box, use commercial NaNH 2 and B 10 h 14 As the initial reactant, NaNH 2 and B 10 h 14 Grind in a mortar and mortar for 30 minutes at a molar ratio of 2:1 until well mixed.
[0044] Take about 0.15g of a well-mixed sample and place it in a volume of 0.7cm 3 Inside the stainless steel reaction crucible, sealed. The stainless steel reaction crucible after sealing the sample was heated in a muffle furnace under the following conditions: 100°C for 10 hours; 200°C for 10 hours; 300°C for 10 hours; 400°C for 10 hours. After the temperature dropped to room temperature, the reaction crucible was taken out, opened in an Ar protection glove box, and the sample was recovered.
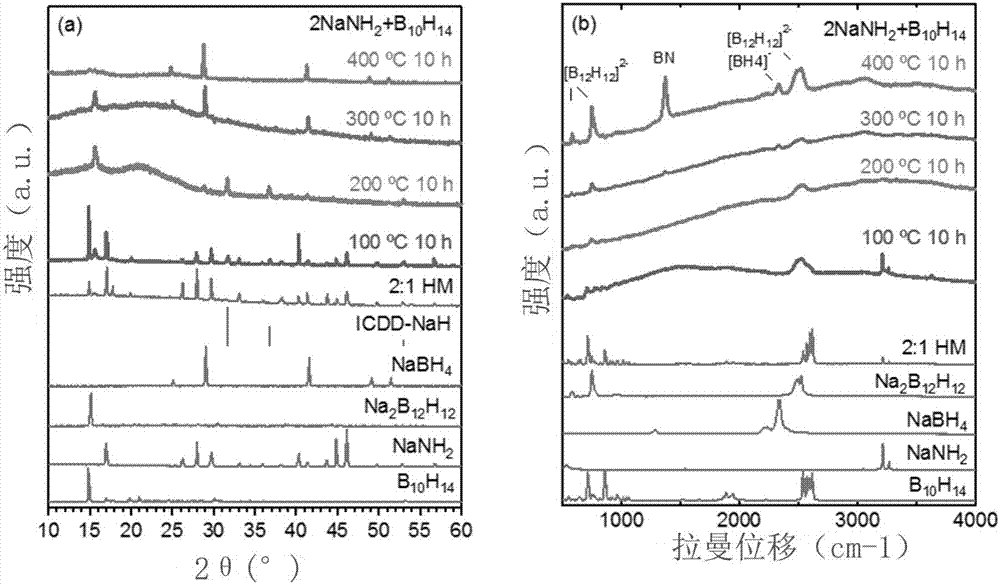
[0045] image 3 is the XRD and Raman diagram of the reaction product, Na 2 B 12 h 12 more than NaBH 4 . In addition to the ratio of raw materials, the reaction conditions can also significantly affect the product. The reaction did not occur at 100 °C, but partially ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com