Mercury ion analysis fluorescent probe and preparation method and application thereof
A technology of fluorescent probes and mercury ions, which is applied in the analysis of materials, fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of insufficient response speed, complex synthesis, and insufficient selectivity, and achieve good stability and simple synthesis , the effect of reducing the impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: preparation formula 5 and formula 8 compounds
[0037] Dissolve 11.2 g of hexamethylenetetramine shown in formula 4 in 20 mL of trifluoroacetic acid, then add 2.16 g of p-cresol shown in formula 3, the molar ratio of the two is 4:1, 100 °C Heating to reflux for 6 hours, and shading treatment, after the reaction, the product was added to ice water, and after the solid was precipitated, suction filtration was performed to obtain the pure product Formula 5.
[0038]
[0039] Formula 3 Formula 4 Formula 5
[0040] Take 656 mg shown in Formula 5 and dissolve in 20 mL of absolute ethanol, then add 500 mg of aminobenzenethiol shown in Formula 6, the molar ratio of the two is 1:1, stir at room temperature, and add dropwise during the stirring process 1184 mg of 37% hydrochloric acid and 2720 mg of 30% hydrogen peroxide were reacted for 30 min, and then filtered by suction to obtain the pure product Formula 8.
[0041]
[0042] Formula 6 Formula 5 Formula...
Embodiment 2
[0044]
[0045] Formula 8 Formula 9 Formula 2
[0046] (Scheme 1) Dissolve 374 mg (1 mmol) of the compound of formula 8 prepared in Example 1 in 10 mL of dichloromethane, add 129 mg (1 mmol) of DIPEA, and then add 173 mg (1 mmol) of benzene thiochloroformate The ester was refluxed at 50° C. for 10 h, and then subjected to suspension evaporation using a rotary evaporator to obtain a solid, which was the crude product of the compound represented by Formula 2. If you want to get a purer product, you can use a mixed system of dichloromethane and petroleum ether (for example, v / v, 1:1) to obtain 423.3 mg of an orange pure product by silica gel column chromatography. The rate is 83%.
[0047](Scheme 2) Dissolve 374 mg (1 mmol) of the compound of formula 8 prepared in Example 1 in 10 mL of dichloromethane, add 387 mg (3 mmol) of DIPEA, and then add 519 mg (3 mmol) of benzene thiochloroformate The ester was refluxed at 50° C. for 10 h, and then subjected to suspension evaporati...
Embodiment 3
[0054] Using the compounds of Scheme 5, probes were prepared. Weigh 5 mg of the compound represented by formula 2 into a colorimetric tube, add 1 mL of dichloromethane, shake well to dissolve the probe, and then dilute to 10 mL with absolute ethanol to prepare a 1 mM probe. Needle stock solution.
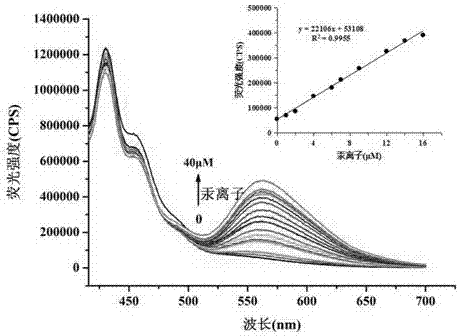
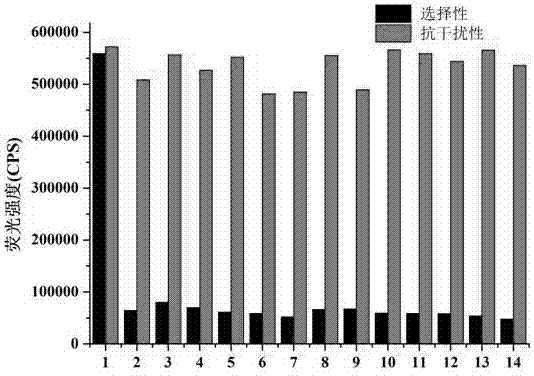
[0055] The prepared probe was used for detection, and the fluorescence detection method was as follows: add 5 mL of absolute ethanol to the colorimetric tube, pipette 50 µL of the probe stock solution (1 mM) into the colorimetric tube, add 2- Add 3mL of distilled water, then add 0.5mL of 4-hydroxyethylpiperazineethanesulfonic acid (HEPES), then dilute to 10mL with distilled water, pipette 200 µL of mercury ion solution (1 mM) into the colorimetric tube. Shake well, and after 20 min, measure the fluorescence spectrum with a fluorescence spectrophotometer (Horiba FluoroMax-4).
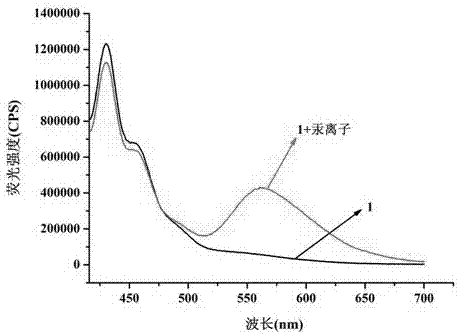
[0056] Test results such as figure 1 shown.
[0057] figure 1 It is the fluorescence spectrum of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com