pH sensitive hydrogel as well as preparation method and application thereof
A hydrogel, sensitive technology, applied in the directions of application, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effects of easy industrial production, overcoming potential safety hazards, and controllable slow-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh an appropriate amount of sodium hyaluronate with a number average molecular weight of 15KDa and dissolve it in water, add excess sodium periodate, stir and react for 2 hours at room temperature in the dark, add appropriate amount of ethylene glycol, continue stirring for 15 minutes, and transfer the reaction mixture to the intercepting Dialyze in deionized water for 72 hours in a dialysis bag with a molecular weight of 0.6 kDa, changing the water every four hours. The dialyzed sample was freeze-dried to obtain a white powder product, ie, formylated sodium hyaluronate, with a formylation rate of 5%.
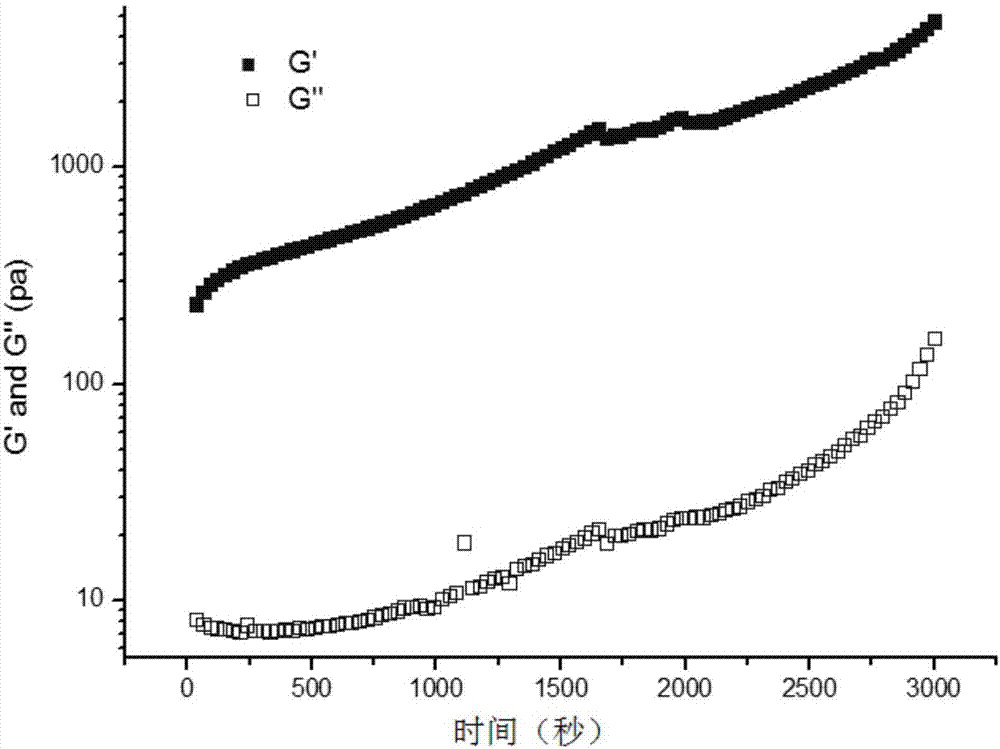
[0038] Add 5g of formylated sodium hyaluronate (formylation rate of 5%) and 1g of four-arm amino-modified polyethylene glycol (Laysan) with a number average molecular weight of 600KDa, add 14g of sterile deionized water, and shake and mix rapidly at 37°C Evenly, after 22 seconds, 20 g of a transparent and uniform pH-sensitive hydrogel was obtained.
[0039] The scann...
Embodiment 2
[0041] Weigh an appropriate amount of sodium hyaluronate with a number average molecular weight of 300KDa and dissolve it in water, add excess sodium periodate, and stir for 2 hours in the dark at room temperature, add an appropriate amount of ethylene glycol, continue stirring for 15 minutes, and transfer the reaction mixture to the intercepting Dialyze in deionized water for 72 hours in a dialysis bag with a molecular weight of 0.6 kDa, changing the water every four hours. The dialyzed sample was freeze-dried to obtain a white powder product, ie, formylated sodium hyaluronate, with a formylation rate of 5%.
[0042] Add 10g of formylated sodium hyaluronate (formylation rate of 5%) and 1g of polyethylene glycol (Laysan) modified with amino groups at the four-arm end of =6.5), at 37° C., oscillated rapidly and mixed evenly, and after 25 seconds, 55 g of a transparent and uniform pH-sensitive hydrogel was obtained.
[0043] The scanning electron microscope images of the pH-sen...
Embodiment 3
[0045] Weigh an appropriate amount of sodium hyaluronate with a number-average molecular weight of 700KDa and dissolve it in water, add excess sodium periodate, stir and react for 2 hours in the dark at room temperature, add appropriate amount of ethylene glycol, continue stirring for 15 minutes, and transfer the reaction mixture to the intercepting Dialyze in deionized water for 72 hours in a dialysis bag with a molecular weight of 0.6 kDa, changing the water every four hours. The dialyzed sample was freeze-dried to obtain a white powder product, ie, formylated sodium hyaluronate, with a formylation rate of 5%.
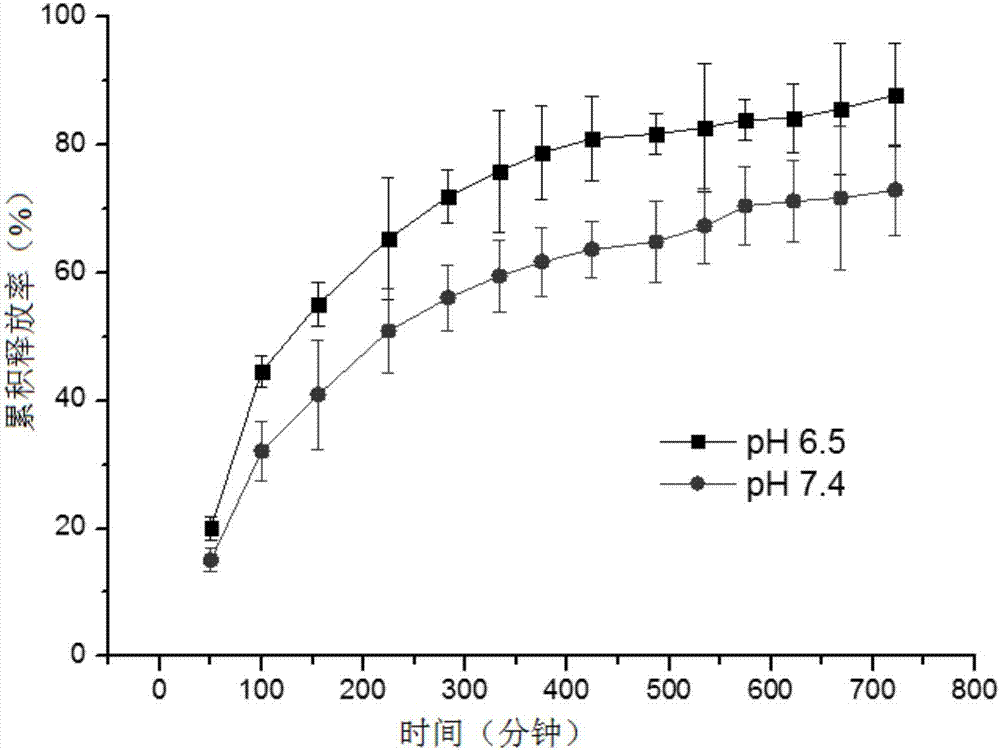
[0046] Add 0.1g of formylated sodium hyaluronate (formylation rate of 5%) and 1g of four-arm amino-modified polyethylene glycol (Laysan) with a number average molecular weight of 800KDa to add 9.9g of disodium hydrogen phosphate-sodium dihydrogen phosphate The buffer solution (pH=7.4) was shaken and mixed rapidly at 37° C., and after 30 seconds, 11 g of a transparent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com