Slow-release transdermal patch containing isosorbide mononitrate and application thereof
An isosorbide dinitrate, sustained-release transdermal technology, applied in the directions of heterocyclic compound active ingredients, cardiovascular system diseases, pharmaceutical formulations, etc. Frequent dosing and other problems to achieve the effect of improving drug safety, small changes in color and stickiness, and avoiding interference and degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: The sustained-release transdermal patch formula of isosorbide mononitrate is as follows:
[0048]
[0049] The preparation method is as follows:
[0050] ①Dissolve isosorbide mononitrate, azone, and stearyl alcohol in ethyl acetate, add silicone pressure-sensitive adhesive and mix evenly. ②Apply the mixed glue on the backing material with an area of 10000cm2, heat and dry , and then covered with a protective layer, ③ cut according to the required size, packaged, and then made into a slow-release transdermal patch containing isosorbide mononitrate. Embodiment 2: The sustained-release transdermal patch formula of isosorbide mononitrate is as follows:
Embodiment 2
[0050] ①Dissolve isosorbide mononitrate, azone, and stearyl alcohol in ethyl acetate, add silicone pressure-sensitive adhesive and mix evenly. ②Apply the mixed glue on the backing material with an area of 10000cm2, heat and dry , and then covered with a protective layer, ③ cut according to the required size, packaged, and then made into a slow-release transdermal patch containing isosorbide mononitrate. Embodiment 2: The sustained-release transdermal patch formula of isosorbide mononitrate is as follows:
[0051]
[0052] The preparation method is as follows:
[0053] ① Dissolve isosorbide mononitrate and stearyl alcohol in ethyl acetate, add silicone pressure-sensitive adhesive and mix well.
[0054] ②Apply the mixed glue on the backing material with an area of 10000cm2, heat and dry, and then cover with a protective layer.
[0055] ③Cut according to the required size, pack, and then make a transdermal patch containing isosorbide mononitrate.
Embodiment 3
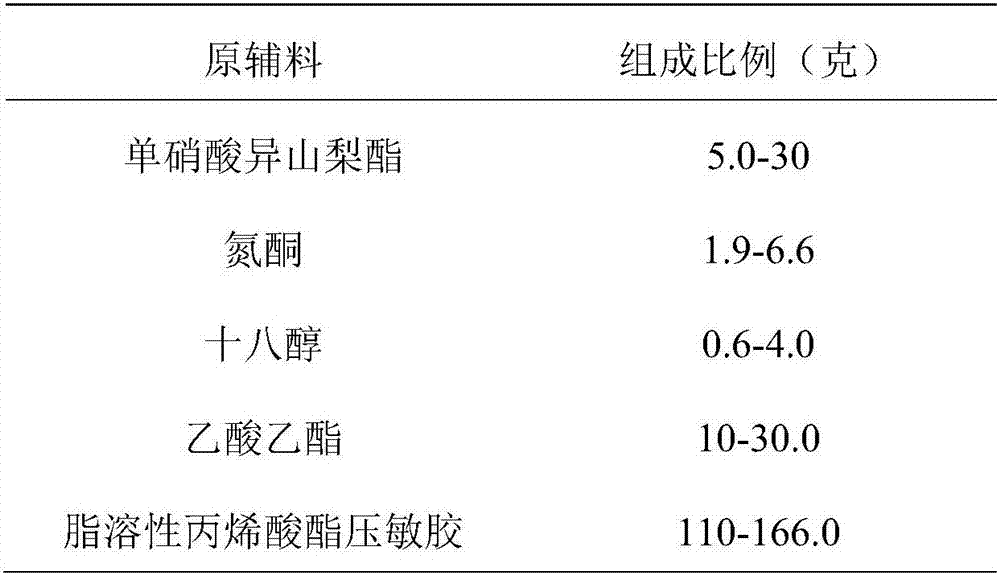
[0056] Embodiment 3: The sustained-release transdermal patch formula of isosorbide mononitrate is as follows:
[0057]
[0058]
[0059] The preparation method is as follows:
[0060] ①Dissolve isosorbide mononitrate, azone, and stearyl alcohol in ethyl acetate, add fat-soluble acrylate pressure-sensitive adhesive and mix evenly. ②Apply the mixed glue on the backing material with an area of 10000cm2, heat and dry, and then covered with a protective layer, ③ cut according to the required size, packaged, that is, to make a slow-release transdermal patch containing isosorbide mononitrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com