Steroidal saponin derivatives, and preparation method and application thereof
A technology of steroidal saponin and derivatives, applied in the field of steroidal saponin derivatives, which can solve the problems of high toxicity, low pharmacological activity, and low product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
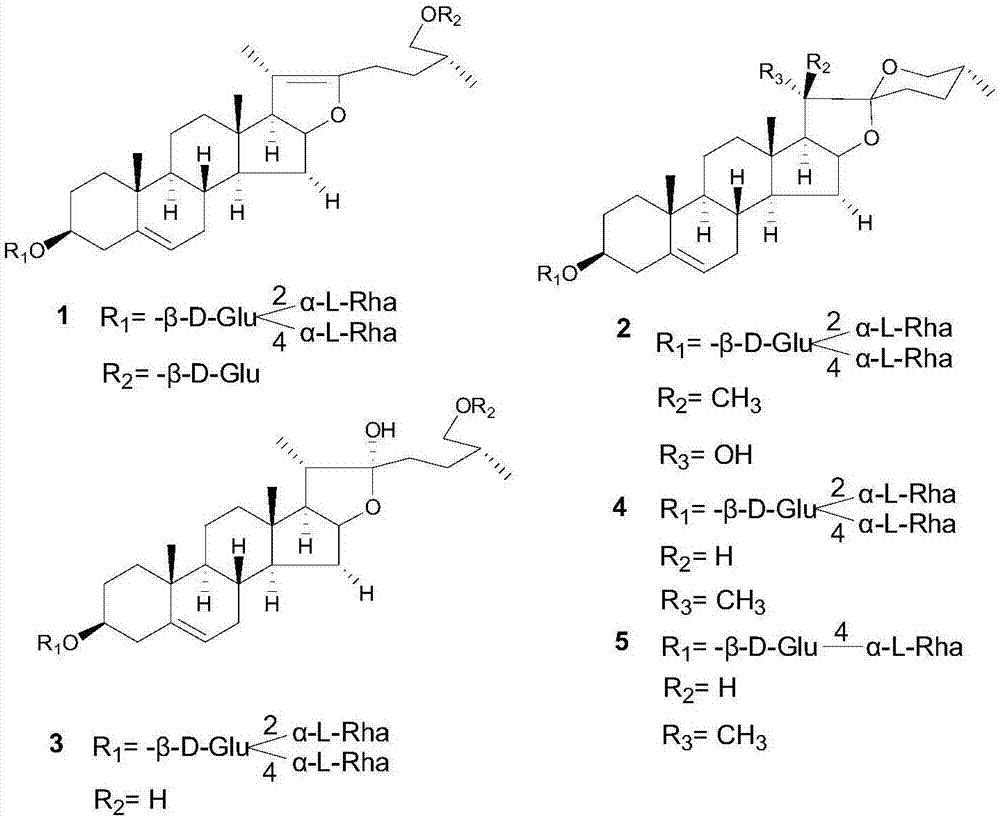
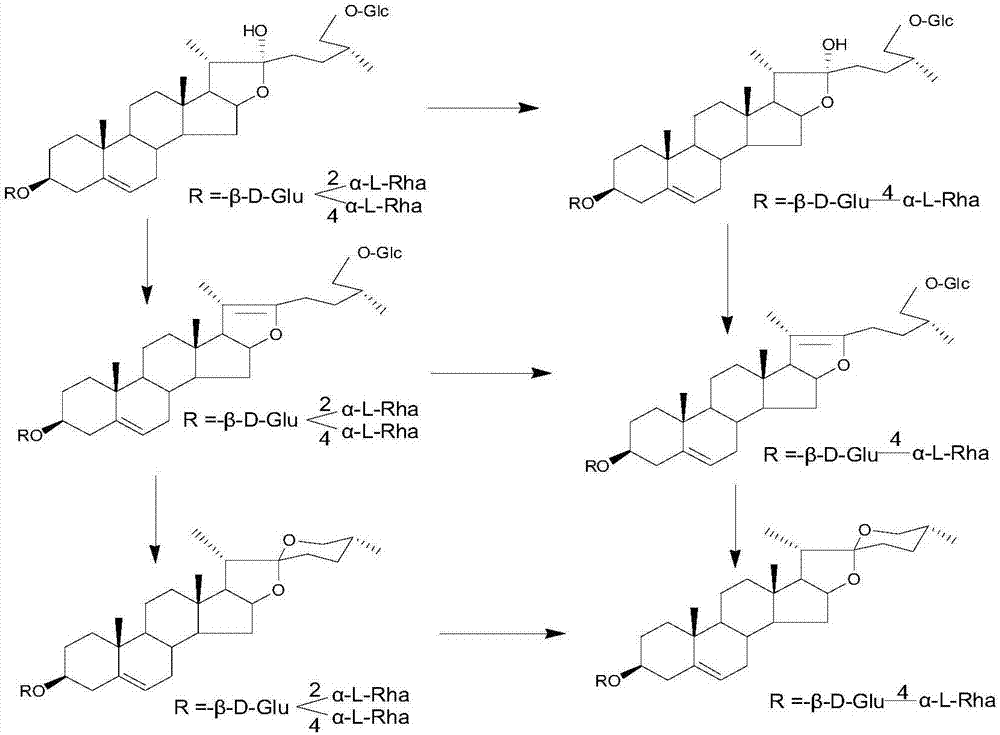
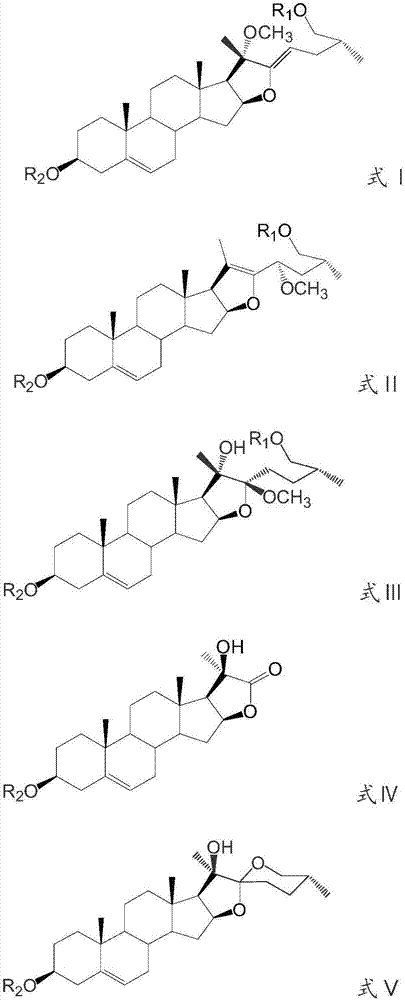
[0047] Preparation and identification of embodiment 1 steroidal saponin derivatives
[0048] 1. Expansion and extraction of strains
[0049] First inoculate the bacteria in the test tube (Chaetomium olivaceum CGMCC 3.3604) in a 250mL Erlenmeyer flask for 2 days, then transfer the bacteria in the Erlenmeyer flask into 17 1L flasks with a plastic dropper in a triangular flask. The medium used in the experiment was PDA medium. The shaker condition is 28°C, 160r / min;
[0050] After continuing to cultivate for 2 days, the substrate pseudoprotodioscin was added. The amount added is 2.5g (dissolve 2.5g of pseudoprotodioscin with 17mL of methanol, and then add 1mL to each Erlenmeyer flask).
[0051] The reaction was terminated 4 days after the addition of the substrate: an equal amount (400 mL) of n-butanol was added to each bottle of medium for ultrasonic extraction for 30 min, and repeated three times. The extract was concentrated by rotary evaporation. A total of 19.32 g of c...
experiment example 1
[0071] In vitro antitumor activity experiment of experimental example 1 steroidal saponin derivatives
[0072] In vitro anti-tumor activity experiments show that the anti-tumor activity of compounds 1-5 prepared in Example 1 of the present invention is significantly better than that of pseudoprotodioscin or compounds with similar structures.
experiment example 2
[0073] In Vitro Cytotoxicity Test of Experimental Example 2 Steroidal Saponin Derivatives
[0074] In vitro cytotoxicity test data prove that the toxicity of compounds 1-5 prepared in Example 1 of the present invention is significantly lower than that of pseudoprotodioscin or structurally similar compounds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com