A recombinant adeno-associated virus gene therapy vector and medicine for treating patients with type 2 achromatopsia
A viral vector and gene technology, applied in gene therapy, the introduction of foreign genetic material using vectors, viruses/phages, etc., can solve the problems of incurable diseases and ineffectiveness of inherited retinal diseases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Construction of embodiment 1.pTR-CBA-AT2R intron 1-hCNGA3 plasmid
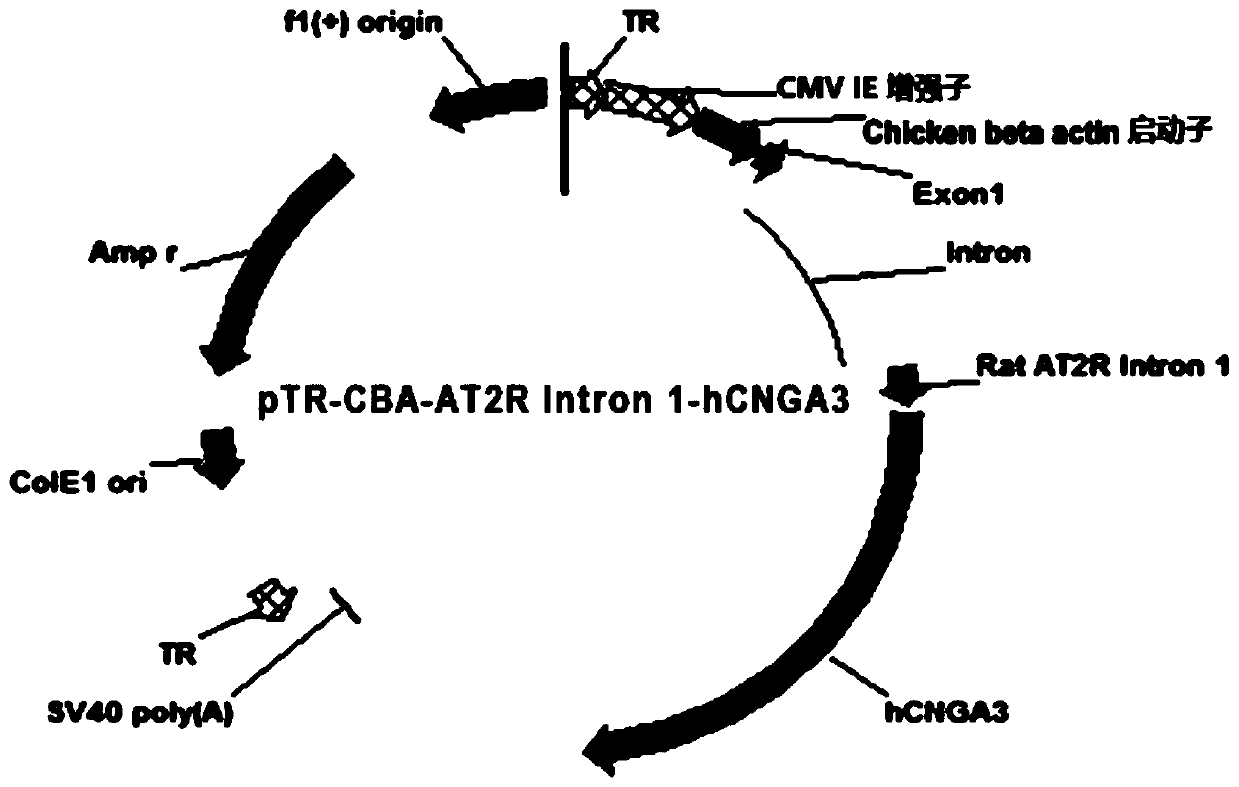
[0046] The cDNA (SEQ ID NO:1) of human CNGA3 (hCNGA) was cloned downstream of the CBA (chicken beta actin) promoter (SEQ ID NO:3) with the CMV IE enhancer (SEQ ID NO:5) in between And the unique rat AT2R (angiotensin II type 2 receptor) Intron 1 (SEQ ID NO: 2), can enhance the long-term expression of the transgene hCNGA. The hCNGA cDNA was followed by the SV40 polyadenylation signal (SEQ ID NO:8). The expression cassette is flanked by inverted terminal repeats: ITR5' (SEQ ID NO: 7), ITR3' (SEQ ID NO: 9), and the viral vector also includes ColE1ori (SEQ ID NO: 10), Amp(r) (SEQ ID NO:11) and f1(+) origin (SEQ ID NO:12).
[0047] The characteristic map of pTR-CBA-AT2R intron 1-hCNGA3 plasmid is as follows figure 1 As shown, the starting and ending points of each component are as follows:
[0048] ITR 5' Start: 19 End: 161
[0049] CMV ie enhancer start: 162 end: 542
[0050] Chiken beta-actin promote...
Embodiment 2
[0059] Construction of embodiment 2.pTR-PR2.1-AT2R intron 1-hCNGA3 plasmid
[0060] The method was the same as that in Example 1, the only difference being that the cone cell-specific promoter PR2.1 (SEQ ID NO: 4) was used instead of the CBA promoter.
Embodiment 3
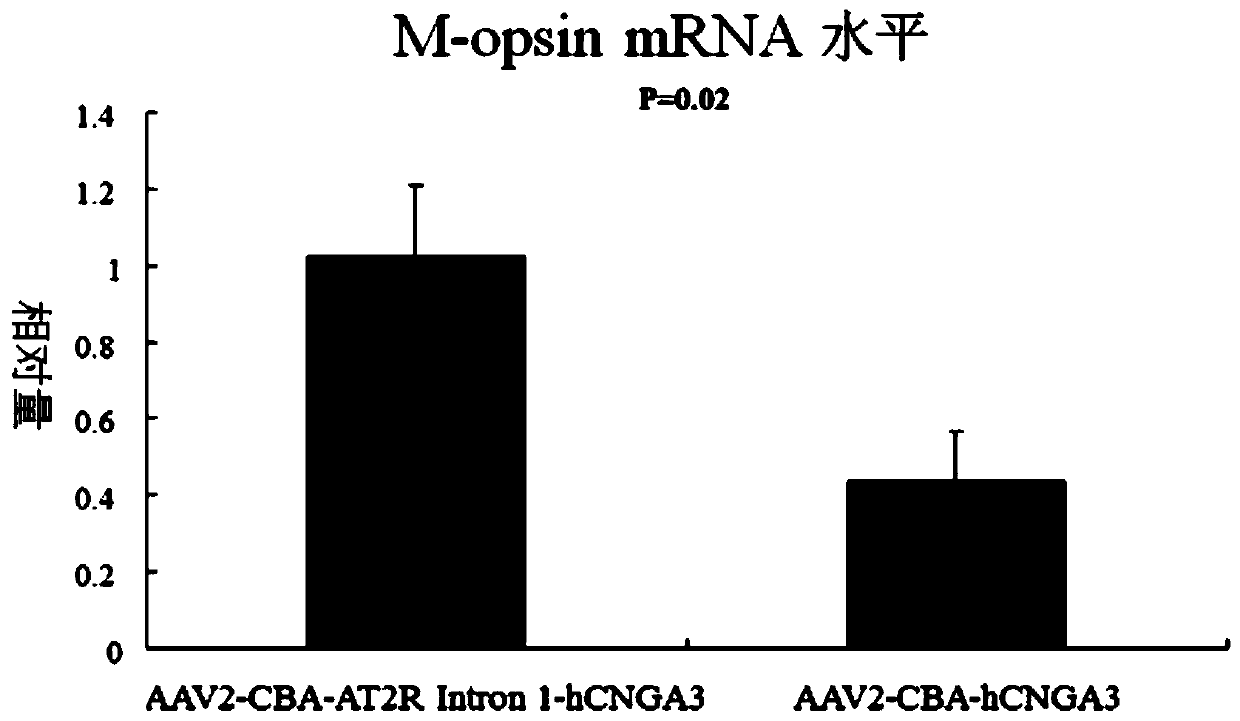
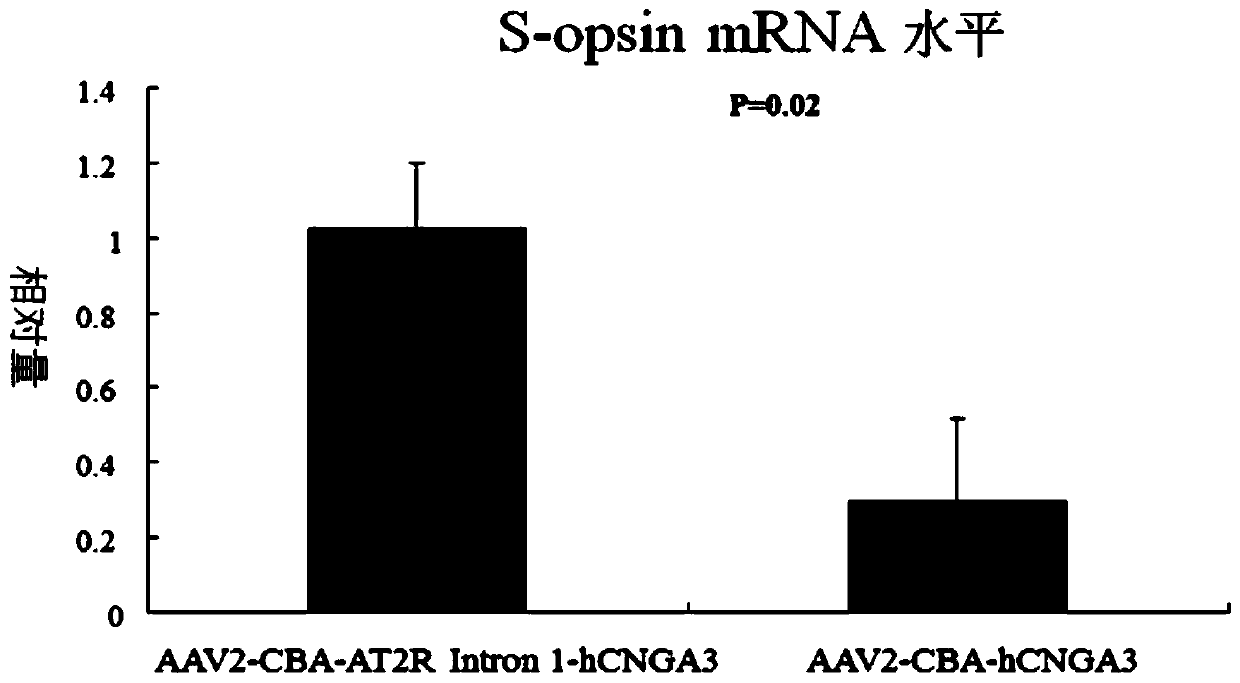
[0061] Embodiment 3. Construction of AAV2-CBA-AT2R intron 1-hCNGA3 viral vector
[0062]Vectors were obtained by plasmid co-transfection method. AAV2-CBA-AT2R intron 1-hCNGA3 recombinant adeno-associated virus vector was initially formed by co-transfecting HEK 293T cells with the helper plasmid containing the AAV2 coat protein gene and the gene that can help AAV replication and the pTR-CBA-AT2R Intron 1-hCNGA3 plasmid . After initial purification with iodine butanol, further purification by ion-exchange chromatography using 5 ml Hitrap Q Sepharose as a filler for fast protein liquid chromatography (the instrument used is Pharmacia AKTA FPLC system (Amersham Biosciences, Piscataway, NJ) .Use the 215mM NaCl of pH8.0 afterwards, rinse the agarose gel column, the recombinant viral vector of peak is collected.After the liquid of collection passes through concentrator (100K concentrater, Millipore), rinse with Tween 20 containing 0.014% The washing concentrator concentrates the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com