Preparation method and application of dibenzofuran compound
A technology of diphenylfuran and compounds, applied in the field of preparation and application of diphenylfuran compounds, can solve the problems of low content of natural active compounds, limited resource sources, complex extraction and separation, etc., to achieve inhibition of proliferation, easy availability of raw materials, and high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
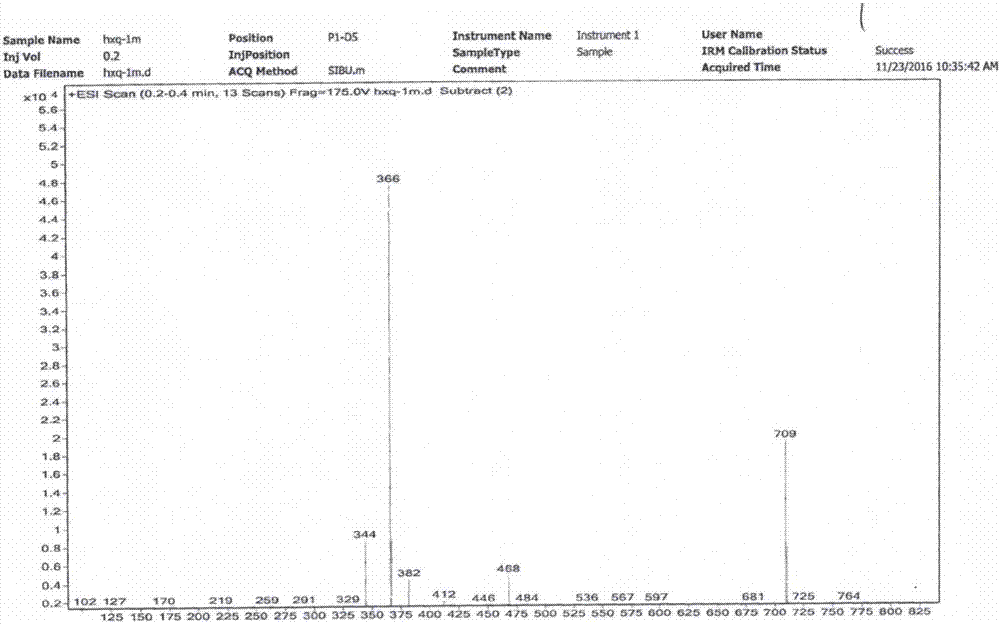
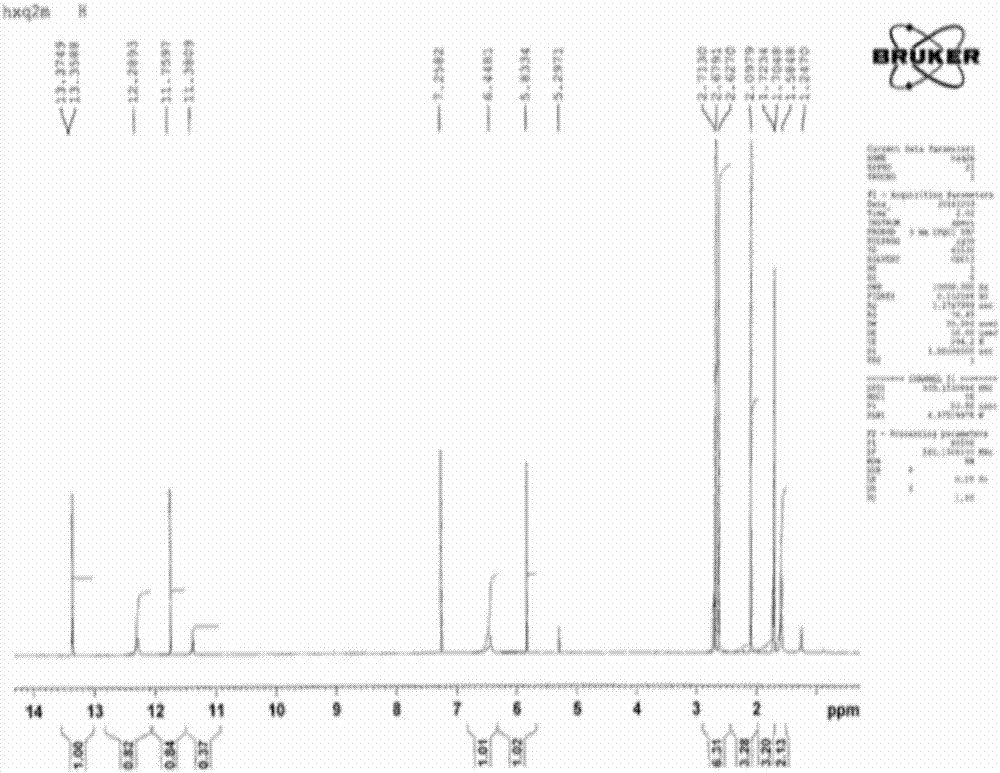
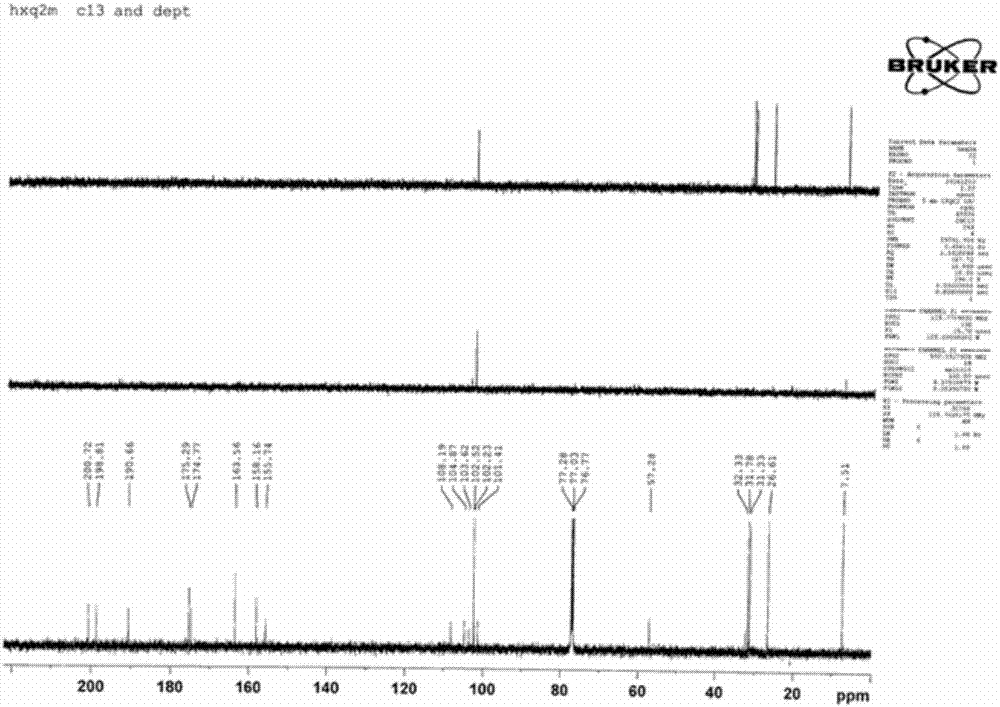
[0057] Diphenylfuran compounds of the present invention: 6-acetyl-2-(1-amino-ethylene)-7,9-dihydroxy-8,9b-dimethyl-9bH-diphenylfuran-1,3- The chemical synthesis and structure identification of diketone (6-Acetyl-2-(1-amino-ethylene)-7,9-dihydroxy-8,9b-dimethyl-9bH-dibenzofuran-1,3-dione).
[0058] Using D-lichenic acid (CAS: 7562-61-0, (+)-Usnic Acid) and hexamethyldisilazane (CAS: 999-97-3, C 6 h 19 NSi 2 or (Me 3 Si) 2 NH) is chemically synthesized as substrates in different solvent media. The results show that using chloroform or ethyl acetate as the solvent medium is the best, the product yield is high, easy to purify, high in purity, and the process is simple. The product yield in methanol and ethanol medium is low, and many impurity by-products will be formed in acetone medium, and the synthesis reaction cannot be carried out in petroleum ether and oily medium. The mol ratio of D-lichenic acid and hexamethyldisilazane is preferably 1:1.7-2.1; the preferred ratio of...
Embodiment 2
[0072] Example 2: Compound 6-acetyl-2-(1-amino-ethylene)-7,9-dihydroxy-8,9b-dimethyl-9bH-diphenylfuran-1 represented by formula (I) , the antitumor effect of 3-diketone (6-Acetyl-2-(1-amino-ethylene)-7,9-dihydroxy-8,9b-dimethyl-9bH-dibenzofuran-1,3-dione), combined antitumor Biological experiment results of action, toxicological safety and anti-tumor mechanism
[0073] The formula (I) compound 6-acetyl-2-(1-amino-ethylene)-7,9-dihydroxyl-8,9b-dimethyl-9bH- Diphenylfuran-1,3-dione has significant, selective, cell-targeted anti-tumor effects, and combined anti-tumor effects with clinical anti-tumor drugs, anti-tumor effects are superior to clinical anti-tumor drugs, and its toxicology Safety and antitumor mechanism of action.
[0074] 1. The effect of diphenylfuran compounds of the present invention on inhibiting and killing human cancer cells in vitro:
[0075] In vitro anti-tumor test method: the diphenylfuran compound of the present invention is dissolved in analytically p...
Embodiment 3
[0107] Example 3: Compound 6-acetyl-2-(1-amino-ethylene)-7,9-dihydroxy-8,9b-dimethyl-9bH-diphenylfuran-1 represented by formula (I) , The mechanism of anticancer action of 3-diketone
[0108] 1. Effect on the growth cycle of cancer cells
[0109] The cell culture test method is the same as the aforementioned in vitro anticancer test method. After the liver cancer cell HepG2 was treated with the diphenylfuran compound represented by formula (I) (at a dose of 1.0 μg / ml) for 48 hours, the cell cycle and apoptosis were detected by flow cytometry (FACS). The positive control group was cisplatin (DDP, 1.5 μg / ml) and 5-fluorouracil (5-Fu, 5.0 μg / ml). The results are shown in Table 4.
[0110] Results: The diphenylfuran compounds represented by the formula (I) can significantly affect the differentiation and cycle of cancer cells, block the cells in the G2 / M phase, and reduce the cells in the G0 / G1 phase and S phase.
[0111] Table 4. Effects on cell cycle (HepG2 cells)
[0112] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com