Preparation method of iprodione
A technology of isofluranil and catalyst, applied in the field of preparation of isoflubenzuron, can solve the problems of uneconomical, low utilization rate, large amount of three wastes, etc., and achieve the advantages of improving the utilization rate of production equipment, shortening production steps, and reducing preparation cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The present invention provides a kind of preparation method of iprodione, comprising:
[0022] Mix N-[[(3,5-dichlorophenyl)amino]carbonyl]aminoacetic acid with an organic solvent, and react under the action of catalyst A to generate 3-(3,5-dichlorophenyl)-2,4- Imidazolidinedione, wherein, catalyst A is the mixture of concentrated sulfuric acid, methanesulfonic acid and p-toluenesulfonic acid;
[0023] React 3-(3,5-dichlorophenyl)-2,4-imidazolidinedione with isopropyl isocyanate under the action of catalyst B and catalyst C to generate iprodione, wherein catalyst B is carbonic acid A mixture of ammonium, sodium bicarbonate, sodium carbonate and liquid ammonia, catalyst C is a mixture of pyridine and triethylamine.
[0024] In the present invention, N-[[(3,5 dichlorophenyl)amino]carbonyl]aminoacetic acid (commonly known as "uric acid" in the industry) is:
[0025]
[0026] 3-(3,5-dichlorophenyl)-2,4C-l imidazolidinedione O is:
[0027]
[0028] The specific chemi...
Embodiment 1
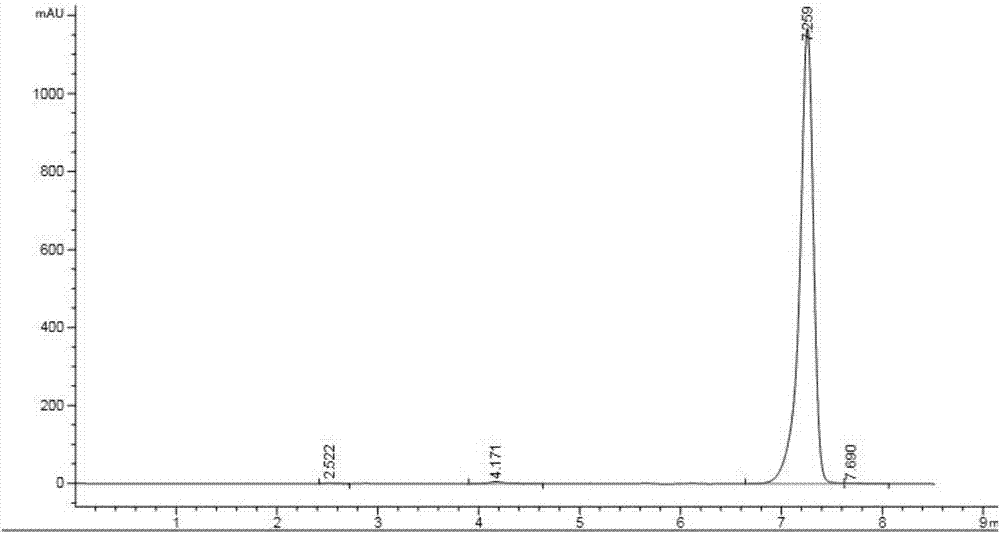
[0040] Add 92g of N-[[(3,5 dichlorophenyl)amino]carbonyl]aminoacetic acid (Mr=263, 0.34mol , 98% industrial product), 400ml organic solvent and 12g catalyst A (0.13 times the mass of uric acid), start stirring, heat the mixture to 90°C, and reflux with water for 4-6h. Add 40g (Mr=85, 0.47mol, 99%, industrial product) of isopropyl isocyanate, 16g of catalyst B (0.17 times the mass of uric acid), and 14g of catalyst C (0.15 times the mass of uric acid) into the reaction flask, and dropwise , heat preservation reaction for 8-10h, after the heat preservation is completed, wash with water, precipitate from the solvent, filter, and dry to obtain 103g of iprodione, with a content of 96.8% and a yield of 88.2%.
[0041] In catalyst A, the mass ratio of concentrated sulfuric acid, methanesulfonic acid and p-toluenesulfonic acid is 1:1:1. In catalyst B, the mass ratio of ammonium carbonate, sodium bicarbonate and sodium carbonate is 1:1:3; in catalyst C, the mass ratio of pyridine and ...
Embodiment 2
[0043]Add 92g of N-[[(3,5 dichlorophenyl)amino]carbonyl]aminoacetic acid (Mr=263, 0.34mol , 98% industrial product), 400ml organic solvent and 12g catalyst A (0.13 times the mass of uric acid), start stirring, heat the mixture to 120°C, and reflux with water for 4-6h. Add 40g (Mr=85, 0.47mol, 99%, industrial product) of isopropyl isocyanate, 16g of catalyst B (0.17 times the mass of uric acid), and 14g (0.15 times the mass of uric acid) of catalyst C into the reaction flask, and dropwise , heat preservation reaction for 8-10 hours, after the heat preservation is completed, wash with water, precipitate from the solvent, filter, and dry to obtain 105 g of iprodione, with a content of 97.5% and a yield of 90.6%.
[0044] In catalyst A, the mass ratio of concentrated sulfuric acid, methanesulfonic acid and p-toluenesulfonic acid is 1:1:1. In catalyst B, the mass ratio of ammonium carbonate, sodium bicarbonate and sodium carbonate is 1:1:3; in catalyst C, the mass ratio of pyridin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com