Zinc porphyrin complex used for electrocatalysis oxygen evolution reaction, and preparation method thereof
A technology of oxygen evolution reaction and complexes, which is applied in organic chemical methods, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., to achieve high yield, easy access to solvents, and time-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This embodiment relates to a method for preparing 5,10,15,20-tetracyanophenylporphyrin, which specifically includes the following steps:
[0036] in N 2 Under atmosphere, add 0.565g p-cyanobenzaldehyde to 500mL anhydrous CH 2 Cl 2 , stirred for 20 minutes to remove the air contained in the solution. Then add 0.3ml of pyrrole to the above mixture and stir well. After refluxing at 130°C for 30 minutes, add 52.5 μL of BF 3 ·OEt 2 (0.85 nmol), and protect the reaction mixture from light with tin foil. After stirring for 2 hours, 0.78 g of p-chlorobenzoquinone (0.37 mmol) was added as a solid, and the solution was refluxed for 4 hours and slowly cooled to room temperature. The residue was separated and purified by silica gel column chromatography, and CHCl 3 Elution afforded (CNTCPP). The NMR data of the ligand obtained in this embodiment are as follows: 1 H NMR (CDCl 3 )δ8.83(s, 8H), 8.35(d, 8H), 8.10(d, 8H).
Embodiment 2
[0038] This embodiment relates to a preparation method for preparing a porphyrin zinc complex, specifically comprising the following steps:
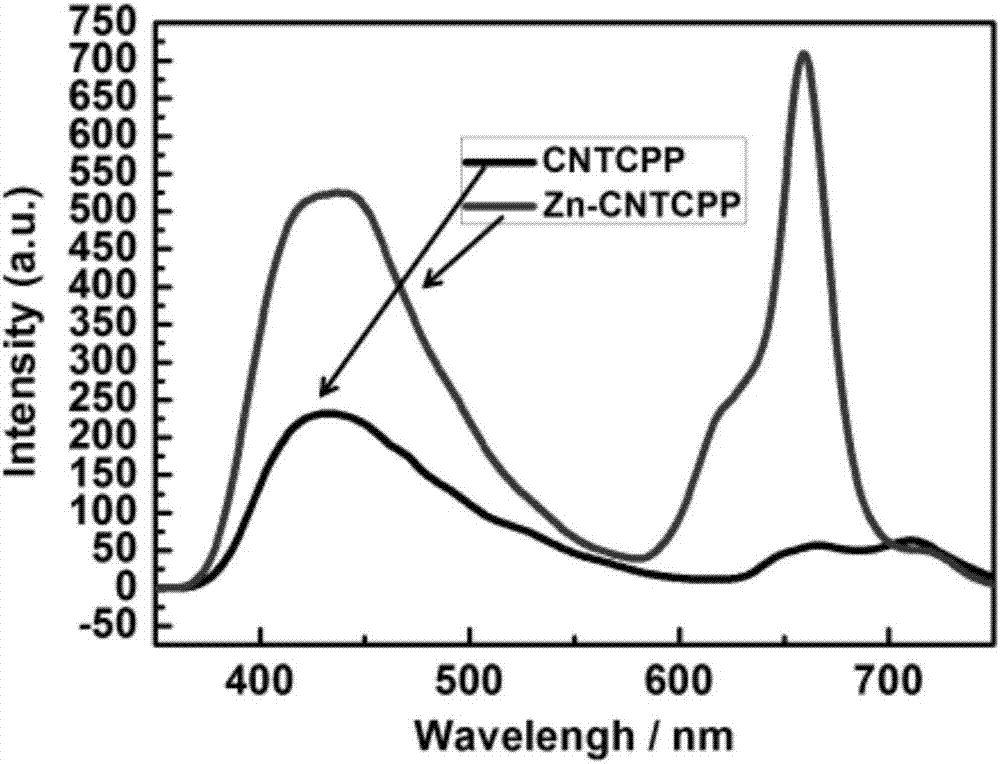
[0039] Take 20mg (0.028mmol) of 5,10,15,20-tetracyanophenylporphyrin prepared in the examples, and 20mg (0.15mmol) of zinc chloride, mix in DMF and EtOH and prepare according to the volume ratio of 2:1 1 mL of mixed solvent, transferred to a glass tube, sealed, and reacted at 120°C for 3000 min to obtain dark brown crystals, which were collected and filtered, washed with EtOH, and dried at room temperature to obtain Zn-CNTCPP. The yield was 25%. The theoretical content of each element in Zn-CNTCPP is respectively: C, 74.09%, H, 3.11%, N, 14.40%; the measured values are respectively C, 74.52%, H, 3.65%, N, 14.02%.
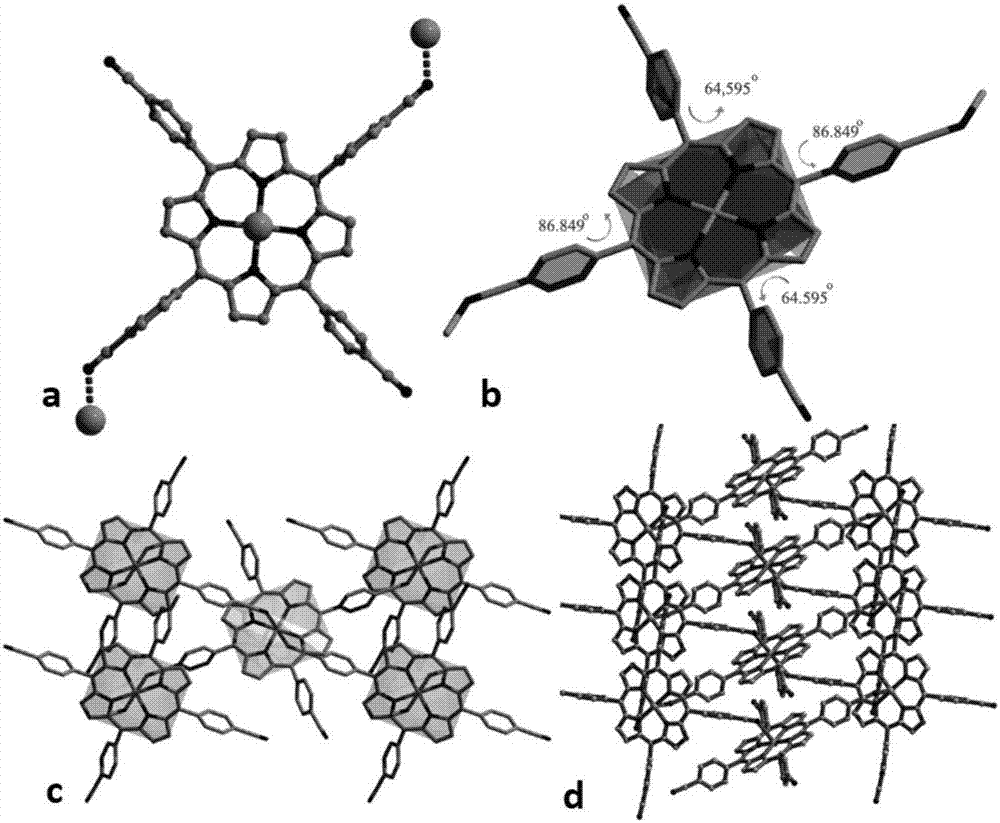
[0040] The crystal structure was collected at room temperature (293K) with an Agilent Xcalibur Eos Gemini single crystal diffractometer. With a graphite monochromator, λ is Absorption correction was performed using a multi...
Embodiment 3
[0050] Embodiment 3 This embodiment relates to the electrocatalytic activity research of porphyrin zinc complex
[0051] Since the zinc ion in the porphyrin center does not form a true coordination bond with the cyano groups in the other CNTCPP ligands, the central Zn 2+ The ion is unsaturated and coordinated, so it can function as a catalytically active center. Therefore, the electrocatalytic activity of Zn-CNTCPP was tested.
[0052] The oxygen evolution reaction activity of the porphyrin zinc complex prepared in Example 2 was detected, and as a comparison, the pure CNTCPP ligand, ZnCl 2 The mixture obtained by manual grinding and mixing with CNTCPP ligand (referred to as mixture A), ZnCl 2 and the CNTCPP ligand obtained by fully reflux mixing in hot DMF (referred to as the mixture B), and the Zn(OH) obtained by the conventional precipitation reaction 2 The electrocatalytic oxygen evolution reaction activity was tested. The specific experimental method is:
[0053] A gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com