Naphthalimide thiophene cyanoethylene polymer as well as preparation method and application thereof
A polymer and compound technology, applied in semiconductor/solid-state device manufacturing, electrical components, circuits, etc., can solve problems such as unsatisfactory stability, and achieve good thermal stability, broad application prospects, and high reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
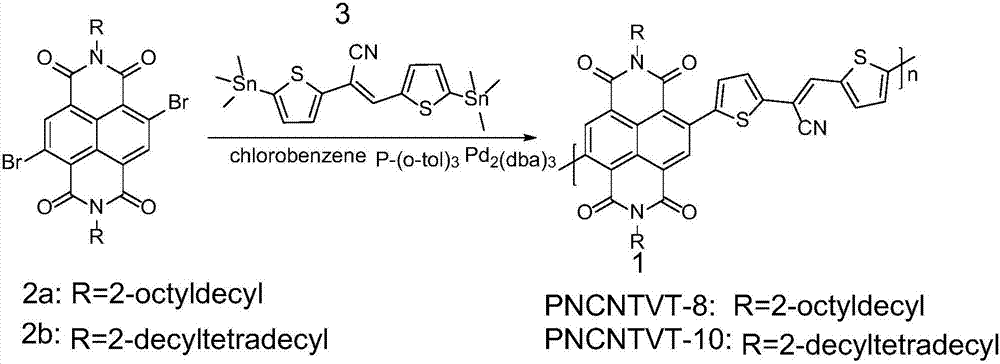
[0041] The synthesis of embodiment 1, polymer PNCNTVT-8
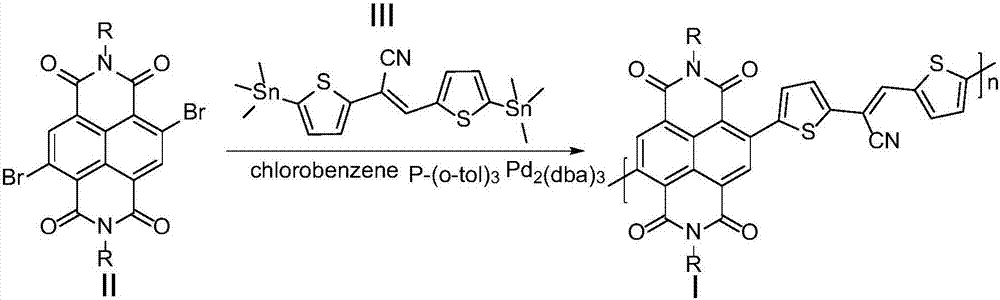
[0042] 4,9-dibromo-2,7-bis(2-octyldodecyl)benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetraketone ( 0.20mmol, 197.01mg) and cis-2,3-bis(5-(trimethyltin) thiophen-2-yl) acrylonitrile (0.20mmol, 108.60mg) shown in formula III, the palladium catalyst is three (diethylene Benzylacetone) dipalladium (6mg, 0.0065mmol), the ligand tris(o-tolyl)phosphine (16.2mg, 0.053mmol) and the organic solvent chlorobenzene (6.0mL) were added to the reaction flask and carried out three times under argon. The freeze-pump-thaw cycle was used to deoxygenate, and then the reaction mixture was heated to 110 °C under the protection of argon to carry out the Stille coupling reaction for 24 h. After cooling, 200 mL of methanol / 6M HCl mixture (v / v 20:1) was added, stirred at room temperature for 2 h, and filtered. The resulting solid was extracted with a Soxhlet extractor. The extraction solvents were methanol, acetone, and n-hexane in sequence,...
Embodiment 2
[0047] Embodiment 2, polymer PNCNTVT-10
[0048] 4,9-dibromo-2,7-bis(2-decyltetradecyl)benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetraketone ( 0.20mmol, 219.46mg) and cis-2,3-bis(5-(trimethyltin)thiophen-2-yl)acrylonitrile (0.20mmol, 108.60mg) shown in formula III, three(dibenzylideneacetone ) Dipalladium (6mg, 0.0065mmol), tri(o-tolyl)phosphine (16.2mg, 0.053mmol) and chlorobenzene (6.0mL) were added to the reaction flask, and three freeze-pump-thaw cycles were carried out under argon After deoxygenation, the reaction mixture was heated to 110 °C under the protection of argon to perform Stille coupling reaction for 24 h. After cooling, 200 mL of methanol / 6M HCl mixture (v / v 20:1) was added, stirred at room temperature for 2 h, and filtered. The resulting solid was extracted with a Soxhlet extractor. The extraction solvents were methanol, acetone, and n-hexane in sequence, each for 24 hours, and finally extracted with chloroform to obtain 182.26 mg of the target polymer,...
Embodiment 3
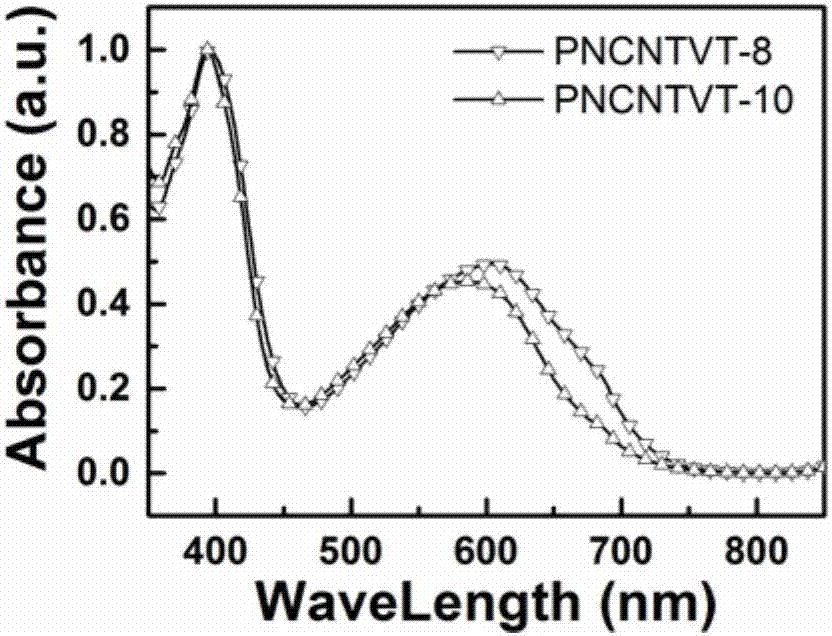
[0053] Spectral performance test of embodiment 3, polymer PNCNTVT-8 and polymer PNCNTVT-10
[0054] image 3 and Figure 4 Be respectively the chloroform solution (concentration is 0.005mmol / L) of the polymer PNCNTVT-8 and polymer PNCNTVT-10 prepared by embodiment 1 and embodiment 2 and the ultraviolet-visible absorption spectrum figure of film, from image 3 It can be seen that there are two absorption bands in this type of polymer, the main absorption band is a high energy band, and its absorption is at 360 to 470 nm, and the secondary absorption band is a low energy band, and its absorption is at 480 to 750 nm. The strong low-energy band absorption indicates that there is a strong D-A interaction in the polymer molecule. Depend on Figure 4 It can be seen that the absorption curve of the film has a red shift to a certain extent compared with that of the solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com