Diarylethene organic photochromic material based on furan as well as preparation method and application thereof
A photochromic material, diarylethene technology, applied in the field of materials science, can solve the problems of high cost and complex synthesis route, and achieve the effects of excellent fatigue resistance, high yield and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 (the synthesis of organic photochromic material DFC):
[0031] Synthesis of compound DFC
[0032]
[0033] The preparation method of 4-bromo-5-methylfuran-2-carbaldehyde (compound 2) can be found in literature (Sysoiev D, Fedoseev A, Kim Y, et al.Synthesis and Photoswitching Studies of Difurylperfluorocyclopentenes with Extendedπ‐Systems[J].Chemistry–A European Journal, 2011, 17(24):6663-6672.)
[0034] Preparation method of 5-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)furan-2-carbaldehyde (compound 3). 250ml three-necked bottle with N 2 Replace gas three times, N 2 Under protection, 9.45g compound 2 (50mmol), 19.05g biboronic acid pinacol ester (75mmol), 14.7g potassium acetate (150mmol) and 1.83g PdCl were added successively. 2 dppf (2.5 mmol). Finally, DMF after strict deoxygenation was added. The reaction was stirred at 80°C for 48 hours. Chromatography (eluent PE:EA=10:1) gave 9.8 g of a light yellow solid with a yield of 83%. 1 H...
Embodiment 2
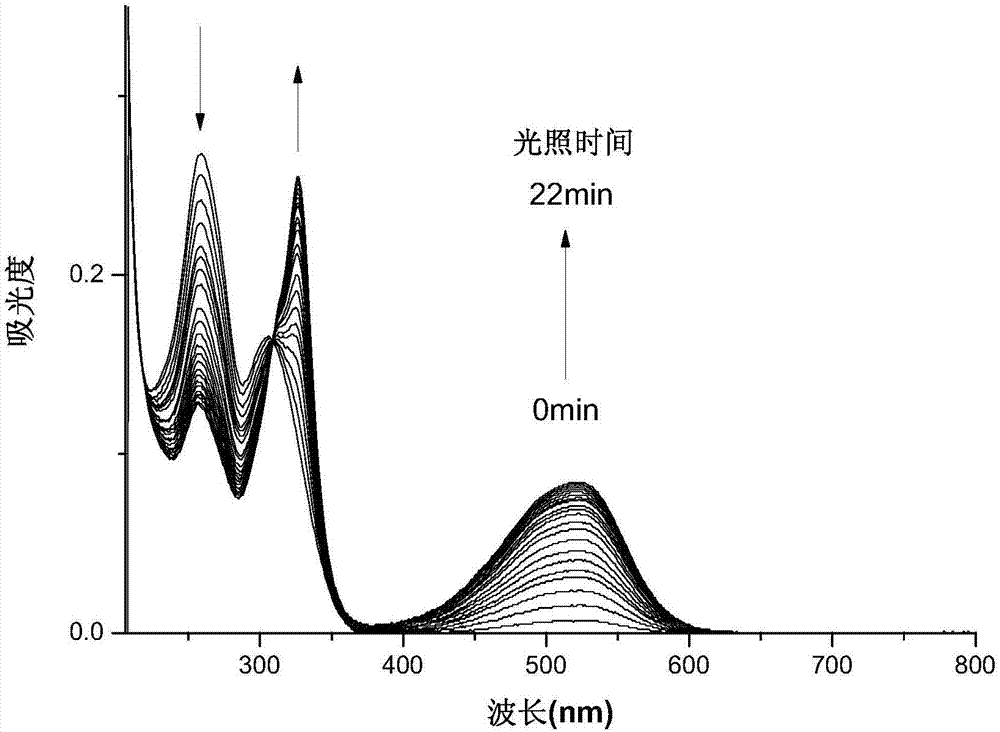
[0036] Embodiment 2 (photochromic performance of organic photochromic material DFC):
[0037] The compound DFC obtained above was dissolved in acetonitrile to make a concentration of 10 μmol L -1 acetonitrile solution. Add 2.5mL of the solution to be tested into a 1cm×1cm×4cm quartz cuvette with stirring, and use a 365nm monochromatic light source according to different time lengths (1min, 2min, 3min, 4min, 5min, 6min, 7min, 8min, 9min , 10min, 11min, 12min, 13min, 14min, 15min, 16min, 17min, 18min, 19min, 20min, 21min, 22min) to irradiate the solution to be tested, and adopt a UV-visible spectrophotometer to measure the absorption spectrum, the result is as follows figure 1 shown. Under the irradiation of 365nm ultraviolet light, a new absorption peak appeared in the absorption spectrum at 520nm, and gradually increased to reach the photostable state with the prolongation of time, and the color of the solution also changed from colorless to pink. Under the irradiation of v...
Embodiment 3
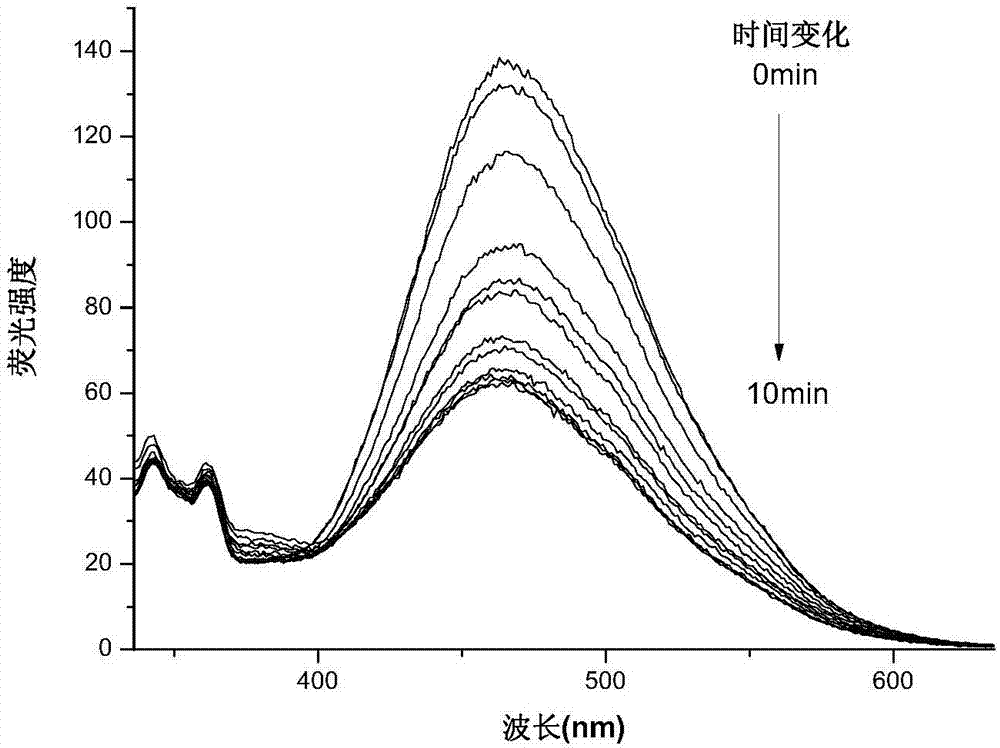
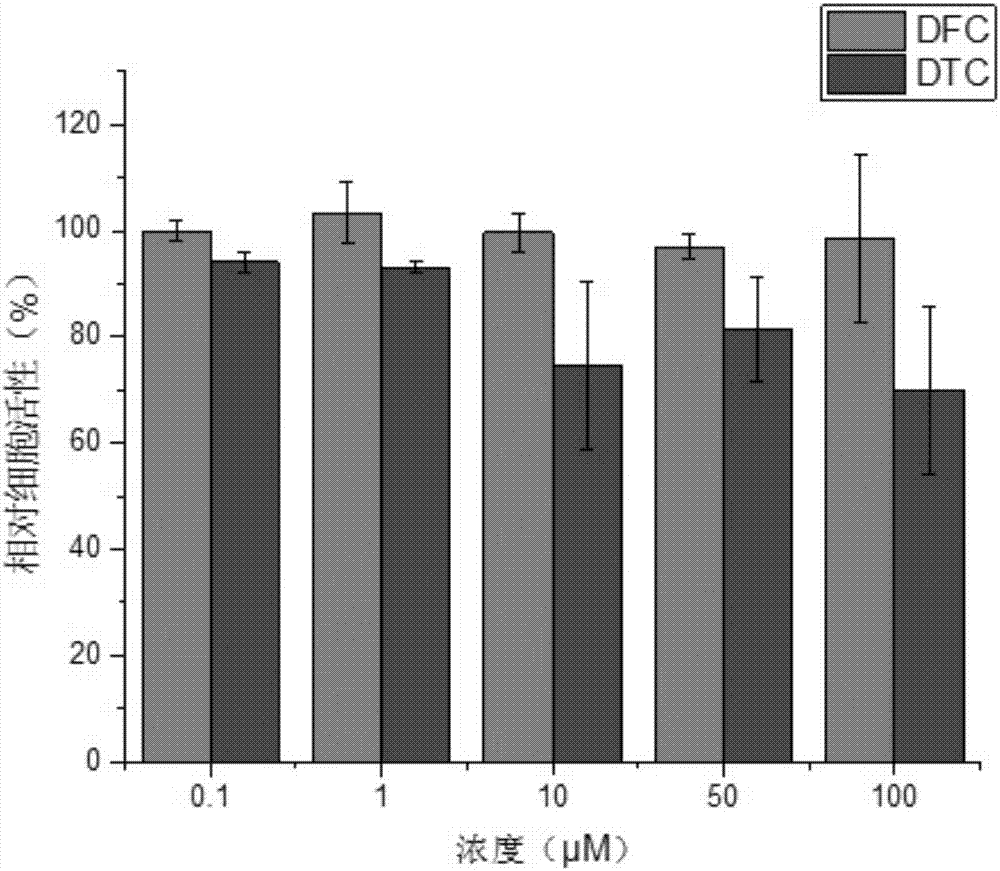
[0038] Embodiment 3 (biological application of organic photochromic material DFC):
[0039] Compounds DFC (1,2-bis(5-formyl-2-methyl-3-furyl)cyclopentene) and DTC (1,2-bis(5-formyl-2-methyl-3-thiophene base) Cytotoxicity of cyclopentene was quantitatively determined by Cell Counting Kit-8 (CCK-8). In the experiment, MC3T3-E1 cells (from the Cell Bank of Shanghai Branch of the Chinese Academy of Sciences) were cultured in MEM-α medium at 1.0×10 4 Cells / well were seeded into 96-well plates. After 12 hours of cell growth, the medium was replaced with new medium (200 μL / well) containing various concentrations of the compounds DFC and DTC. Before performing the CCK-8 assay, replace the medium from the previous step with 200 μL of new medium containing 20 μL of CCK-8 solution, and incubate the cells for a further 48 h. After incubation for 3 hours, the absorbance at 450 nm in each well was measured using a microplate reader (Multiskan Mk 3). Quantitative cytotoxicity was calcula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com