Use of nitrogen-containing heterocyclic radical substituted alkene compounds
A compound and application technology, applied in chemical instruments and methods, methine/polymethine dyes, instruments, etc., can solve the problems of cumbersome imaging operations, luminescent properties staying, and not being widely involved, and achieve great application prospects, imaging Excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
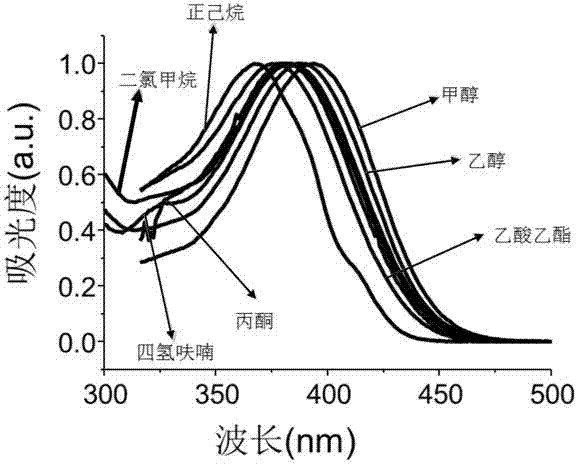
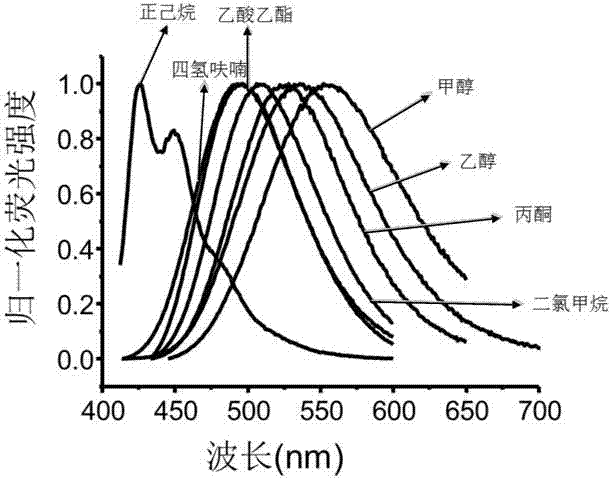
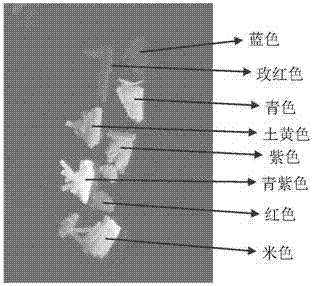
Image
Examples
Embodiment 1
[0217] Embodiment 1, the synthesis (I-2025) of (E)-2-(4-methoxystyryl) quinoline
[0218]
[0219] Mix and dissolve 0.83g 2-methylquinoline and 1.00g p-toluenesulfonamide (TsNH2) in 10ml toluene, raise the temperature to 120°C, then add 0.79g p-methoxybenzaldehyde, and react at 120°C for three days, The reaction was monitored by thin-plate TLC until complete. Stop the reaction, heat and stir, cool to room temperature, suction filter to obtain a solid, wash with ethanol, and recrystallize with ethanol to obtain the target product as a light yellow solid with a yield of 82%.
[0220] 1 H NMR (400MHz, CDCl3) δ8.12(d, J=8.6Hz, 1H), 8.06(d, J=8.5Hz, 1H), 7.85-7.55(m, 6H), 7.49(t, J=7.5Hz , 1H), 7.32(d, J=7.9Hz, 1H), 6.94(d, J=8.7Hz, 2H), 3.86(s, 3H).
Embodiment 2
[0221] Embodiment 2, (E)-N-(4-(2-(quinoline-2-yl) vinyl) phenyl) acetamide synthesis (I-2061)
[0222]
[0223] According to the same process of Example 1, only p-methoxybenzaldehyde is replaced by an equivalent amount of p-acetamidobenzaldehyde, the solid obtained is washed with ethanol, and recrystallized with ethanol, thus obtaining (E)-N-( 4-(2-(quinolin-2-yl)vinyl)phenyl)acetamide, pale yellow solid, yield 89%.
[0224] 1 H NMR (400MHz, CDCl3) δ8.11(d, J=8.6Hz, 1H), 8.07(d, J=8.5Hz, 1H), 7.78(d, J=8.0Hz, 1H), 7.73-7.45(m , 8H), 7.33(d, J=16.3Hz, 1H), 2.19(s, 3H), 1.72(s, 1H).
Embodiment 3
[0225] Embodiment 3, according to the procedure of embodiment 1, just replace 2-methylquinoline with equivalent 2,6-dimethylquinoline and 6-bromo-2-methylquinoline respectively, thus obtain ( E)-N, N-dimethyl-4-(2-(6-methylquinolin-2-yl)vinyl)aniline and (E)-4-(2-(6-bromoquinolin-2 -yl)vinyl)-N,N-dimethylaniline.
[0226] (E)-N,N-Dimethyl-4-(2-(6-methylquinolin-2-yl)vinyl)aniline (I-2063)
[0227]
[0228] 1 H NMR (300MHz, DMSO) δ8.17(d, J=8.6Hz, 1H), 7.83(d, J=8.6Hz, 1H), 7.71(dd, J=18.8, 12.3Hz, 3H), 7.55(d , J=8.6Hz, 3H), 7.17(d, J=16.3Hz, 1H), 6.76(d, J=8.8Hz, 2H), 2.97(s, 6H), 2.48(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com