A kind of iron, cobalt, nitrogen co-doped carbon catalyst and its preparation method and application
A carbon catalyst and co-doping technology, which is applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of single active sites of TM-N-C catalysts, decreased catalytic activity of oxygen reduction, and uneven dispersion of active sites. Achieve excellent oxygen reduction catalytic activity, good dispersion of metal nanoparticles, and excellent mass transfer performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
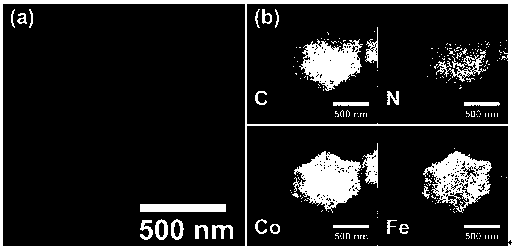
Image
Examples
Embodiment 1
[0035] Preparation of Fe / Co Bimetallic Zeolite Imidazolate Framework Precursors
[0036]Weigh 208.5 mg of ferrous sulfate and 654.8 mg of cobalt nitrate and dissolve them in 160 mL of methanol, and weigh 3.9408 g of 2-methylimidazole and dissolve them in 160 mL of methanol. The above methanol has been passed through N 2 half an hour to remove dissolved O from the solvent 2 , preventing Fe 2+ was oxidized; pour the above salt solution into the 2-methylimidazole solution, stir for 2 min and mix well, at 30 o C.N 2 After standing for 12h under a protective environment, suction filtration, washing, and drying at 80 o Dry under C for 8 h, and the obtained product is the precursor of iron / cobalt bimetallic zeolite imidazolate framework material (named as Fe,Co-ZIF(1:3) according to the feeding ratio of Fe and Co).
[0037] Preparation of iron, cobalt and nitrogen co-doped carbon catalysts
[0038] Take 200 mg of the above iron / cobalt bimetallic zeolite imidazolate framework mat...
Embodiment 2
[0040] Using the preparation process of Example 1, the difference is that the addition of ferrous sulfate becomes 139 mg, the addition of cobalt nitrate becomes 727.5 mg, and the prepared catalyst is Fe, Co, N-CNP (1:5) . Compared with the catalyst prepared in Example 1, the onset potential and half-wave potential of the catalyst are both negative, and the catalytic activity is inferior to that of the catalyst in Example 1.
Embodiment 3
[0042] Using the preparation method of the iron / cobalt bimetallic zeolite imidazolate framework material precursor in Example 1, the only difference is that the addition amount of ferrous sulfate becomes 417 mg, and the addition amount of cobalt nitrate becomes 436.5 mg, wherein Fe 2+ / Co 2+ The molar ratio is 1:1. At this time, the morphology of the synthesized product has been completely deformed, and the regular polyhedral structure is no longer maintained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com