Half-quantity resolution method of racemic mixture of tetrahydroisoquinoline type compound
A technology of tetrahydroisoquinoline and racemate, which is applied in the field of medicine, can solve the problems of high consumption of resolving agent, unsuitability for industrial production, and time-consuming, so as to reduce the amount of resolving agent, save reaction cost, shorten The effect of reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Dissolve 20g of tetrahydropapaverine hydrochloride in 400mL of distilled water, stir until completely dissolved, add ammonia water to dissociate, until no white oil precipitates, add 400mL of toluene and stir well, until the water layer is free of tetrahydropapaverine, use anhydrous sulfuric acid Sodium-dried toluene layer was spin-dried to obtain 17.3 g of tetrahydropapaverine racemate.
[0042] Dissolve tetrahydropapaverine racemate in 173mL of acetonitrile, heat to 70°C, slowly add 3.93g of resolving agent N-acetyl-D-leucine, stir for 20min, add resolving agent N-acetyl-D - 0.522g of phenylalanine, stirred for 35 minutes, slowly lowered to room temperature, and then placed in a refrigerator at 4°C for 24 hours to wait for crystallization.
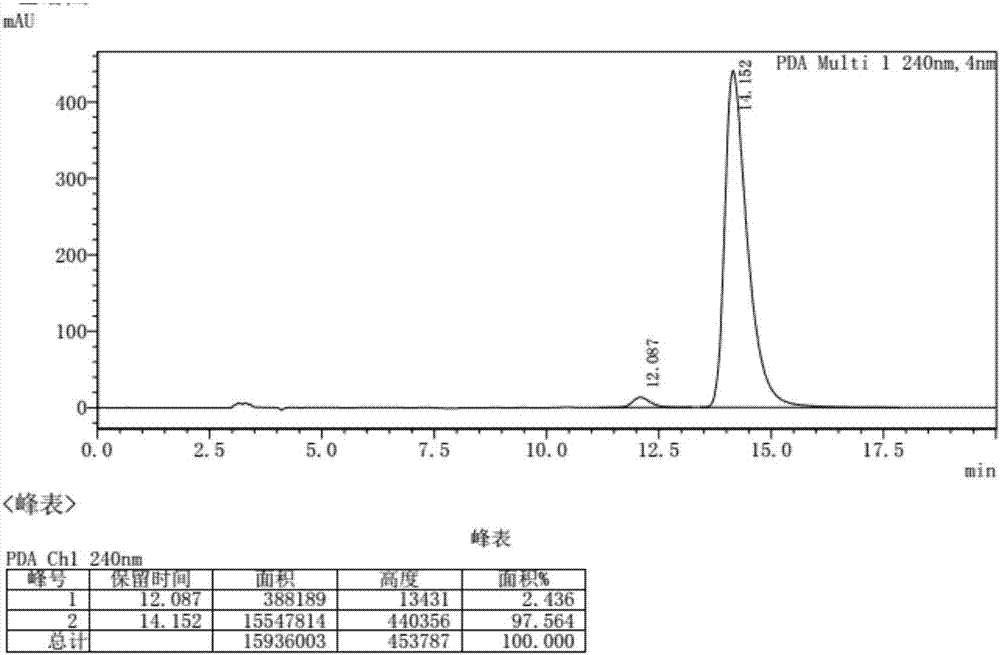
[0043] After the solid precipitated, filter, add hydrochloric acid to convert the product into hydrochloride, and dry to obtain 8.325 g of R-tetrahydropapaverine hydrochloride. The total yield is 83.25%, and the purity is 97.6%. ...
Embodiment 2
[0045] Dissolve 30g of tetrahydropapaverine hydrochloride in 600mL of distilled water, stir until completely dissolved, add ammonia water to dissociate, until no white oily matter precipitates, add 600mL of toluene and stir well, until the water layer is free of tetrahydropapaverine, wash with anhydrous sulfuric acid Sodium-dried toluene layer was spin-dried to obtain 26.1 g of tetrahydropapaverine racemate.
[0046] Dissolve tetrahydropapaverine racemate in 261mL of acetonitrile, heat to 70°C, slowly add 12.56g of resolving agent D-di-p-toluoyl tartaric acid, react for 45min, slowly cool down to room temperature, and place in a refrigerator at 4°C 24 hours, waiting for crystallization.
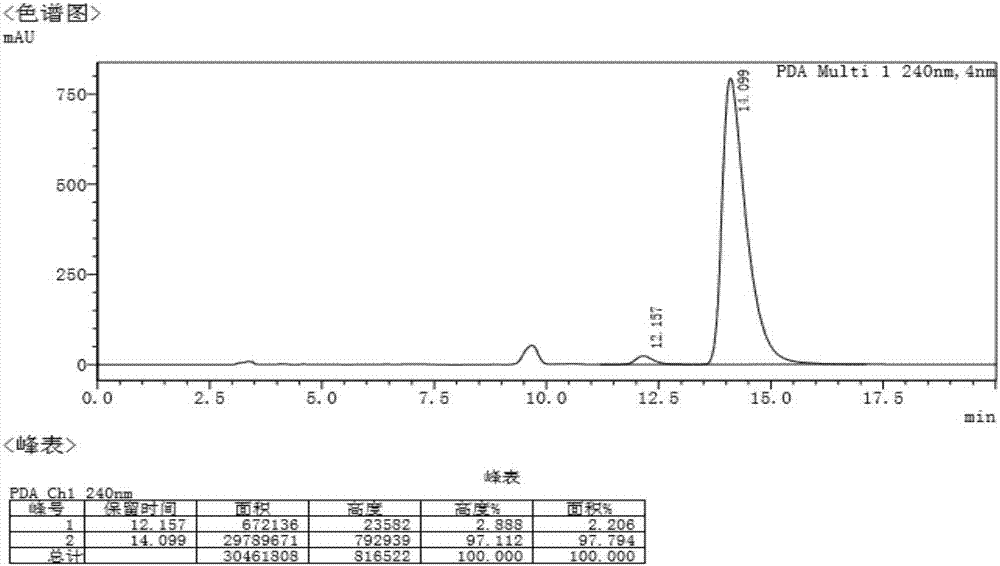
[0047] After the solid is precipitated, filter and dry to obtain the salt of R-tetrahydropapaverine and resolving agent. The obtained salt was converted into R-tetrahydropapaverine hydrochloride, dried and weighed, 12.3g, with a total yield of 82% and a purity of 97.8%. The chromatogram of ...
Embodiment 3
[0049] Dissolve 1.5g of tetrahydropapaverine hydrochloride in 30mL of distilled water, stir until completely dissolved, add ammonia water to dissociate, until no white oil precipitates, add 30mL of toluene and stir well, until the water layer is free of tetrahydropapaverine, use anhydrous The toluene layer was dried with sodium sulfate and spin-dried to obtain 1.32 g of tetrahydropapaverine racemate.
[0050]Dissolve tetrahydropapaverine racemate in 13.2mL acetonitrile, heat to 70°C, slowly add resolving agent N-acetyl-D-phenylalanine 0.379g, react for 45min, slowly cool down to room temperature, and then in 4 ℃ refrigerator for 24 hours, waiting for crystallization.
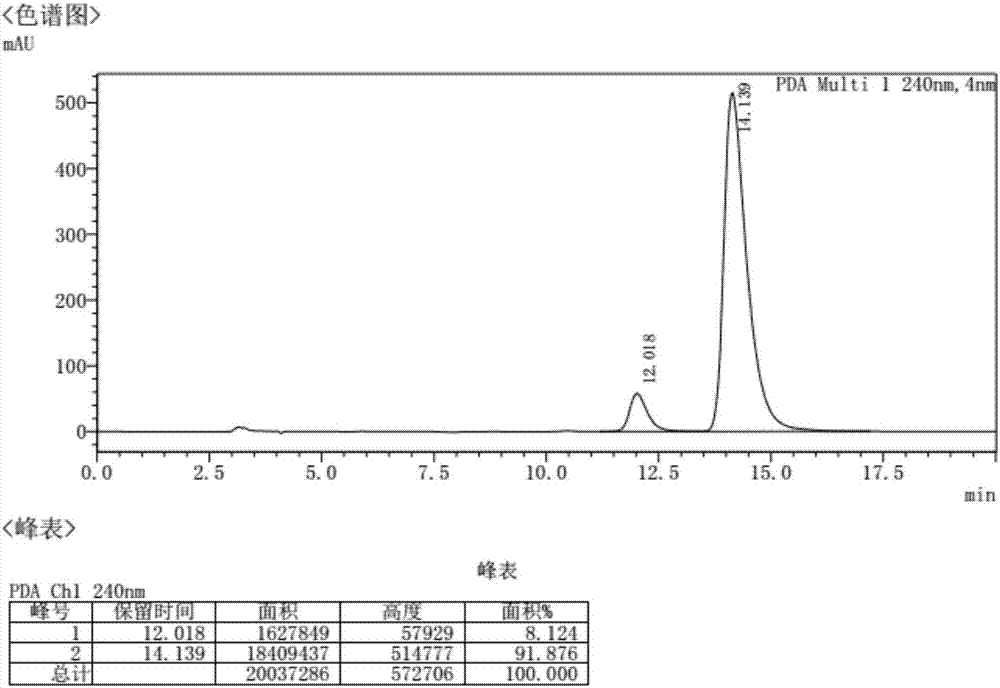
[0051] After solid precipitation, filter and dry to obtain R-tetrahydropapaverine N-acetyl-D-phenylalanine salt, and convert the obtained salt into R-tetrahydropapaverine hydrochloride, totaling 0.60 g. The total yield is 80.1%, and the purity is 91.9%. The chromatogram of the product is as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com