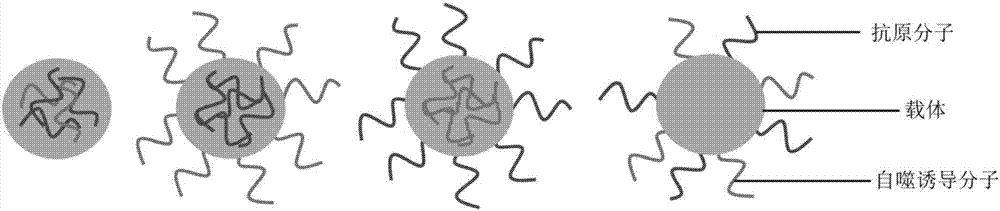
Medicine as well as preparation method and application thereof
A drug and reaction technology, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problem that anti-tumor DNA or peptide vaccines cannot truly enter the clinic, low antigen capture and uptake ability, and unsatisfactory anti-tumor vaccine immune effects, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation of embodiment 1 carrier
[0070] Taking polyurethane compound as an example, its molecular structure is:
[0071]
[0072] The specific method of polymer synthesis is: (1) Weigh 1.2mmol of 1,6-hexanediol diacrylate, 0.9mmol of N,N-dibutylpropylenediamine and 0.1mmol of NH2-mPEG2000, and dissolve them all Disperse in 2ml of dimethyl sulfoxide (DMSO), pass through N after completely dissolving 2 15 minutes, then heated to 50°C and reacted in the dark for 7 days; (2) Take out the reaction product after 7 days, use a dialysis bag with a cut-off of 3500 to dialyze in water for 6-9 hours, and collect the product in the dialysis bag; (3) Freeze-dried, the product was collected after freeze-drying for subsequent experiments.
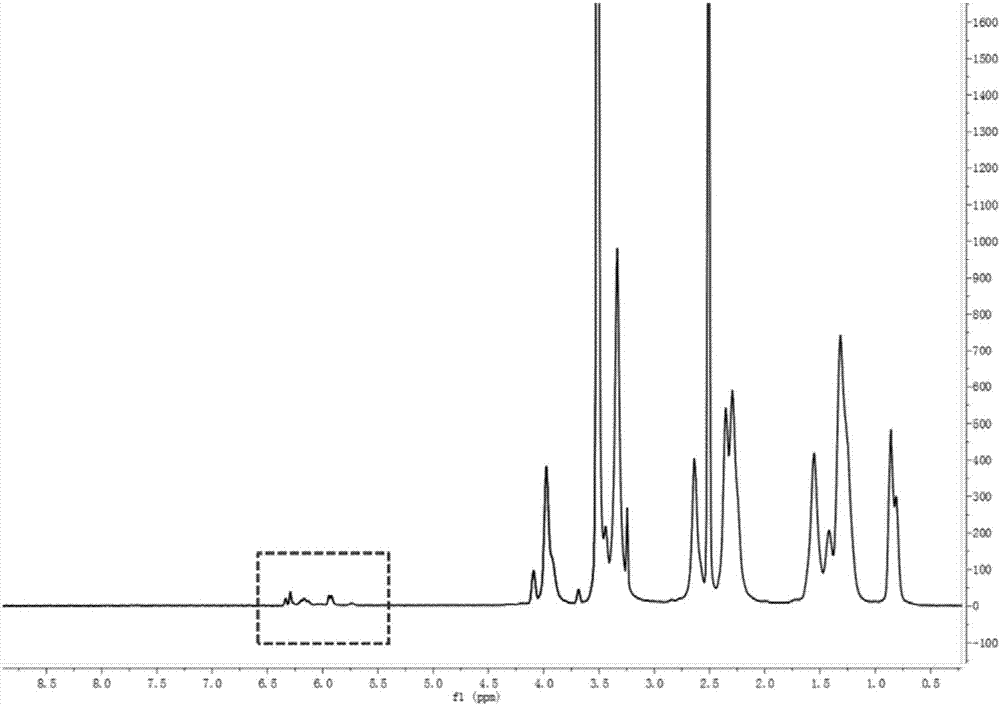
[0073] The NMR structure of the carrier is as figure 2 As shown, the position of the dotted box indicates a double bond, and it can be seen that the vector was successfully constructed.
Embodiment 2
[0074] The preparation of embodiment 2 medicines
[0075] Dissolve the reaction product in Example 1 in DMSO, add excess autophagy-inducing peptide Beclin1, and tumor antigen peptide OVA, and react in the dark at 37°C for 3 days; (2) Take out the reaction product after 3 days, and use a cut-off of The dialysis bag of 3500 was dialyzed in water for 6-9 hours, and the product in the dialysis bag was collected; (3) freeze-dried, and the product was collected after freeze-drying for subsequent experiments. The product is a molecule with autophagy induction function and tumor antigen loading.
[0076] The obtained modified polypeptide molecule, that is, the chemical structure of the drug molecule 3 is as follows:
[0077]
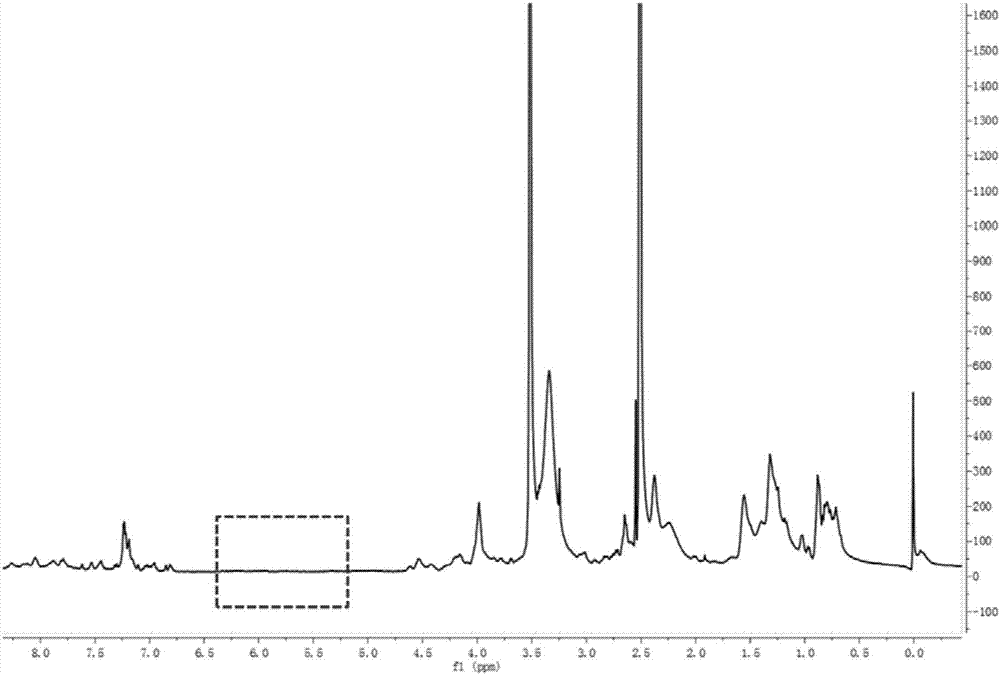
[0078] Its schematic diagram is as follows figure 1 As shown, the NMR spectrum is as image 3 As shown, it can be seen from the figure that the double bond at both ends of the polymer in the original carrier molecule disappears (the dotted box indicates th...
Embodiment 3
[0081] Drugs described in Example 3 improve the induction of autophagy
[0082] First, use the DC2.4 cell line as the object to study the effect of molecularly induced autophagy. Divide DC2.4 cells into 5×10 4 Inoculate confocal dishes at 37 °C and 5% CO 2 Incubate for 12 hours under the conditions; use lipo3000 to transfect the cells, transfect the GFP-LC3 plasmid, and after 6 hours of transfection, replace the medium containing the transfection reagent with complete medium, and culture for another 12-16 hours;
[0083] Add autophagy inducers, inhibitors and the corresponding molecule 3 or control molecule 2 to the transfected cells respectively. After incubation for 12 hours, wash the cells with PBS and observe the GFP fluorescence of the cells using a confocal microscope. The results are as follows: Figure 4 , the level of molecularly induced autophagy can be preliminarily judged by GFP-LC3 fluorescence.
[0084] When the cells were only treated with the carrier molecul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com