Method for manufacturing immunotherapy vaccine
A technology for immunotherapy and vaccines, applied in biochemical equipment and methods, blood/immune system cells, medical preparations containing active ingredients, etc., can solve the problems of low fusion efficiency of dendritic cells and cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] Embodiments of the present invention are described below. The scope of rights of the present invention is not limited by this example.
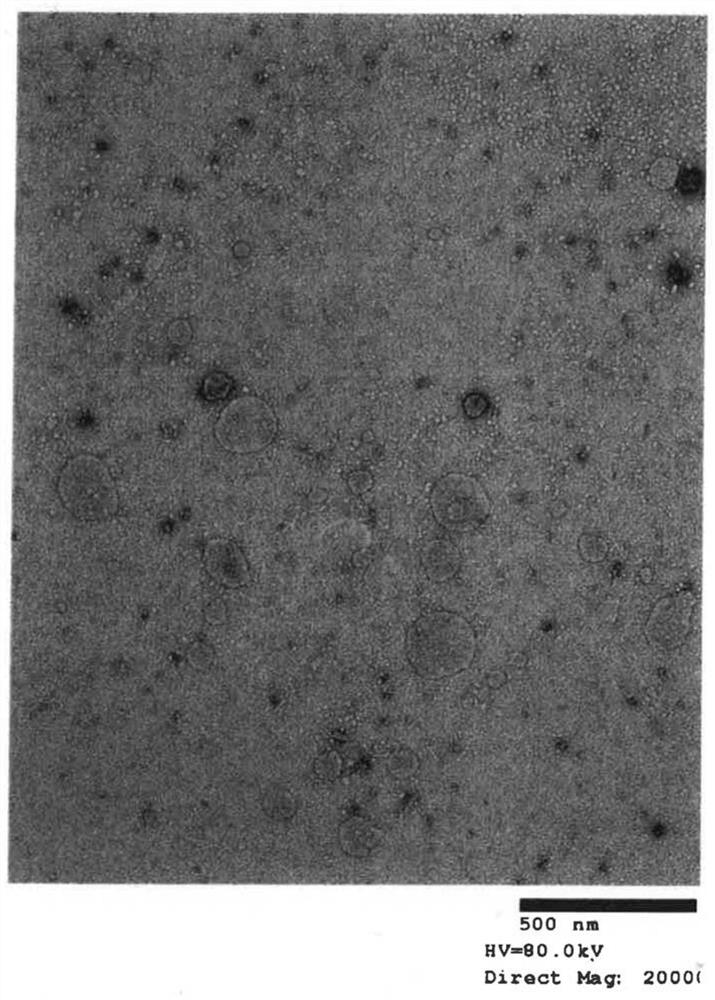
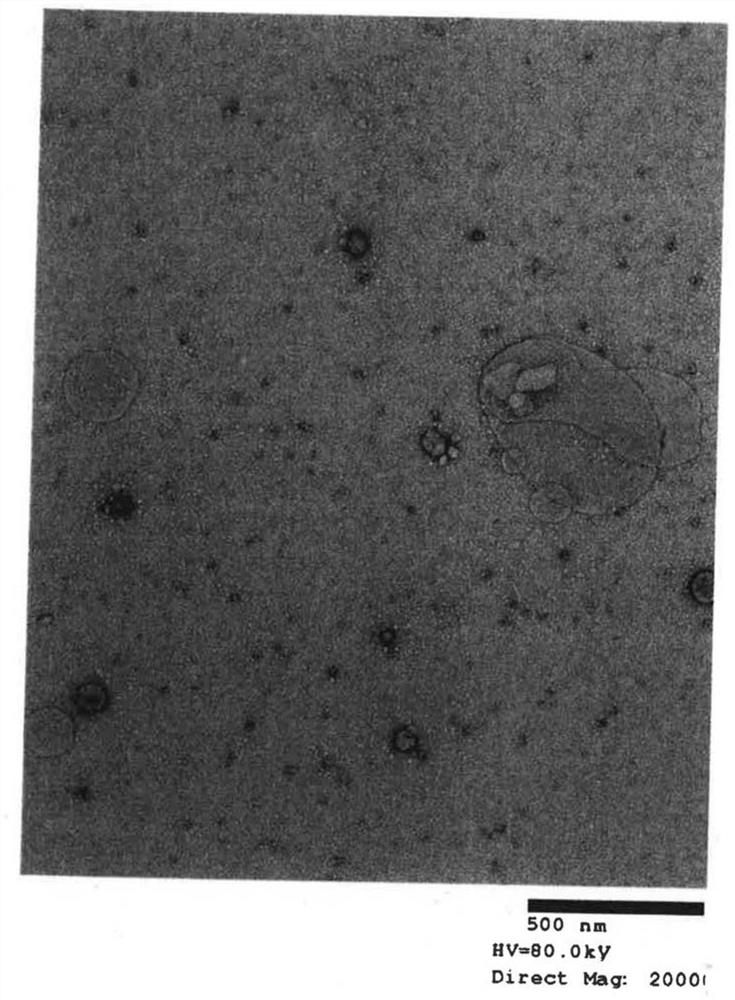
[0042] (A) Extraction of exosomes from cell culture supernatant
[0043] Step 1: After culturing for 2 days in FCS-supplemented medium from which exosomes were removed, extraction was performed from the culture supernatant. For extraction, miRCURY Exosome Cell / Urine / CSF Kit (TAKARA BioCat. 300102) was used as an isolation kit.
[0044] Step 2: Measure the number of exosome particles extracted in step 1.
[0045] Step 3: Exosomes are inactivated by heat treatment (56° C., 30 minutes).
[0046] Exosomes are easily disintegrated by heating the cell membrane. Since the cell membrane disintegration cannot fuse with the dendritic cells, the upper limit temperature and the upper limit time of the heat treatment are determined by changing the heating conditions. The method of determination is based on observation of the membrane structure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com