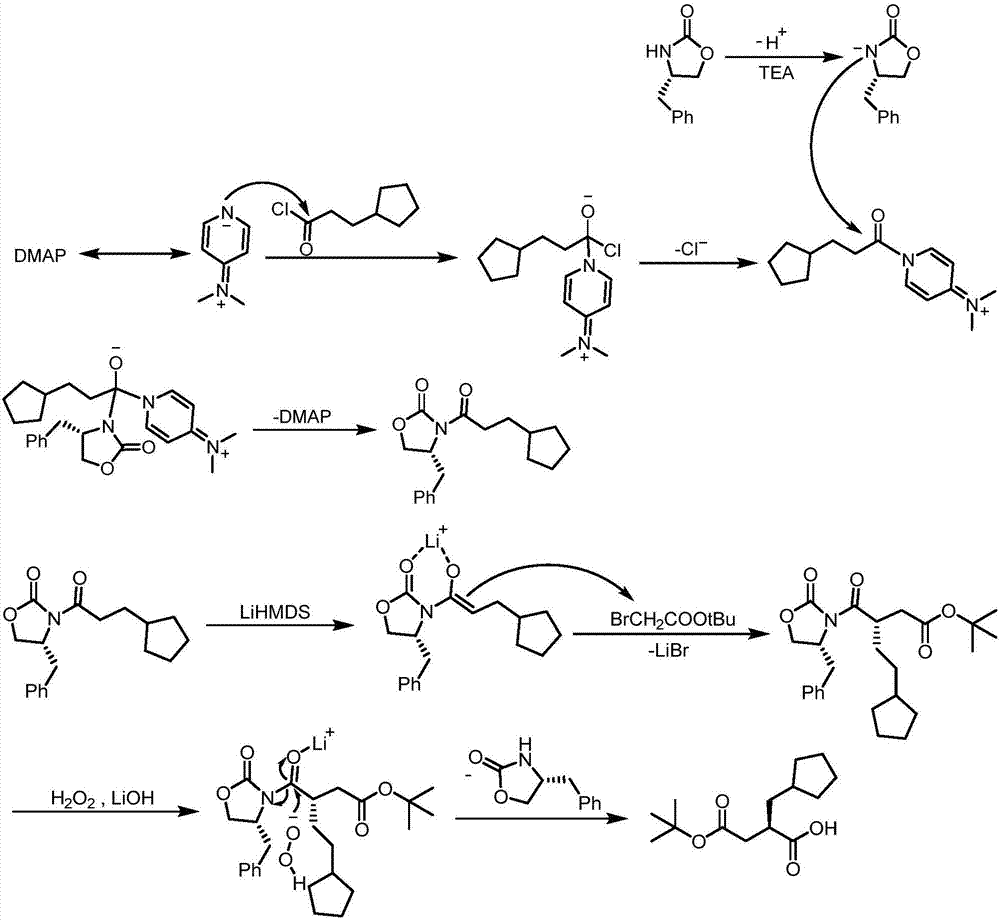
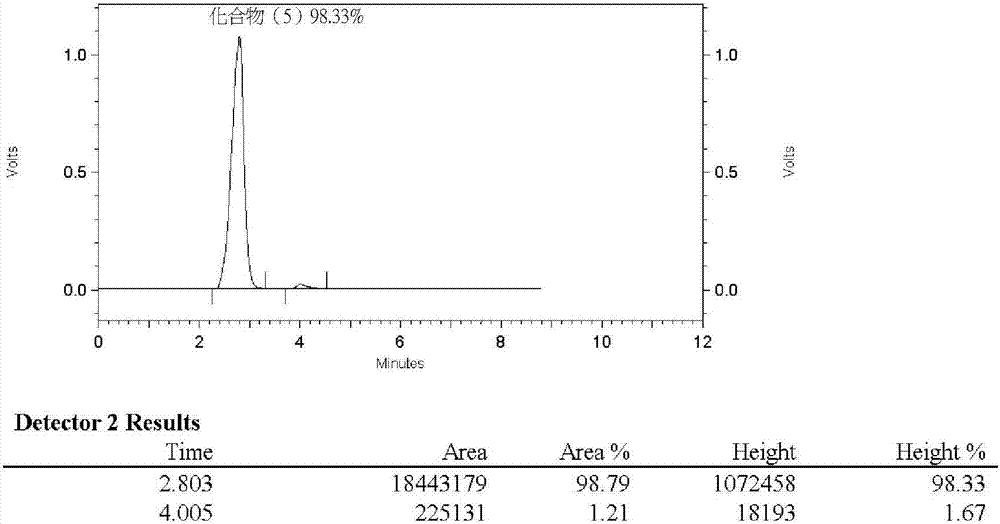
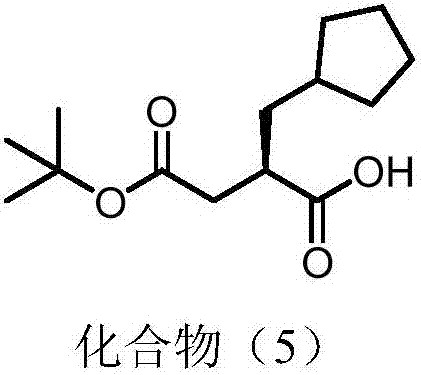
Synthesis method of (R) 4 (tert-butoxy) 2 (cyclopentyl methyl) 4 oxobutyric acid
A technology of pentylmethyl and tert-butoxy, which is applied in the field of synthesis of -4--2--4-oxobutanoic acid, can solve the problem of increasing the cost of process production, reducing the optical purity of products, and increasing reaction costs, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The first step: the synthesis of formula (3) compound (S)-4-benzyl-3-(3-cyclopentylpropionyl)-2-oxazolidinone
[0053] Add compound (S)-4-benzyl-2-oxazolidinone (2) (150.0g, 0.85moL), 4-dimethylaminopyridine (155.1g, 1.27moL), triethyl Amine (128.60g, 1.27moL), dichloromethane (1500mL), cooled to 0-5°C in an ice-water bath, and compound 3-cyclopentylpropionyl chloride (1) (145.80g, 0.89moL) was added dropwise, and the temperature was controlled below 10°C, the dropwise addition was completed, and the reaction was continued for 4.0 hours, followed by TLC (developer: petroleum ether: ethyl acetate = 5:1), and the resulting solid was removed by filtration, and the filtrate was sequentially added to a saturated aqueous solution of sodium bicarbonate (500 mL ), 10% citric acid aqueous solution (2*500mL), washed with saturated brine (500mL), dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure to obtain a crude product, added n-heptane (300mL) fo...
Embodiment 2
[0062] Under the condition that the experimental environment is guaranteed and the experimental operation is basically the same, a series of screenings have been carried out on the solvent used in the first step reaction of Example 1, reaction temperature, alkali and catalyst, and its operation is the same as the first step in Example 1 operation, the filtered results are shown in Table 1 below:
[0063] Table 1
[0064]
Embodiment 3
[0066] Under the condition that the experimental environment is guaranteed and the experimental operation is basically the same, a series of screenings are carried out on the solvent used in the second step reaction of Example 1, reaction temperature, alkali and catalyst, and its operation is the same as the second step in Example 1 operation, the results are shown in Table 2 below:
[0067] Table 2
[0068]
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com